Introduction
Gould (Reference Gould1989) famously advocated a critical role for contingency in the history of life, employing the lineages preserved in the mid-Cambrian Burgess Shale to suggest that the early history of animals could have played out with different patterns of success among various lineages of bilaterians. Other studies have evaluated the uniqueness of discrete evolutionary innovations (Crick Reference Crick1968; Raup and Valentine Reference Raup and Valentine1983; Kauffman Reference Kauffman1995; Gould Reference Gould2002; Vermeij Reference Vermeij2006), in part from interest in the role of contingency versus determinism or predictability in the history of life. This tension between contingent and unique explanations for historical events versus repeated patterns, albeit within the context of particular initial conditions and pathways, is a fundamental issue for understanding patterns and processes in the history of life.
Take two extreme examples. If historical contingency is the dominant mode in the history of life, then attempts to develop a general theory of either macroevolution or microevolution, beyond the most basic assumptions about evolutionary processes, are questionable. Historical narrative may be the most paleontologists can realistically expect to contribute (although this is a view that few readers of Paleobiology are likely to find attractive). In contrast, the nascent field of astrobiology is predicated on sufficient regularities in the nature of life that we can employ our understanding of life on Earth to make predictions about both the probability of life and its nature elsewhere in the universe.
But the history of life includes a mix of deterministic and historically contingent processes (the meaning of contingency will be explored below). Some events, such as the Ediacaran–Cambrian Radiation (ECR) appear to some to be so unique as to defy a uniformitarian explanation. However, some evolutionary biologists have adopted an implicitly uniformitarian approach, denying that there was anything unique about the processes involved in such events. For example, Vermeij (Reference Vermeij2006) examined 23 purportedly unique events in the history of life, comparing them with a suite of 55 innovations that have happened more than once. His analysis suggested that the apparent uniqueness of many evolutionary innovations reflected information loss due to their occurrence early in the history of life rather than true uniqueness. From this Vermeij concluded that many evolutionary innovations are highly deterministic, and something like them would have happened even if not in the same lineage or at the same time. Note that Gould and Vermeij are addressing different questions: Gould was arguing that the long-term success or failure of clades that arose during the ECR was unpredictable, whereas Vermeij was essentially arguing that something like the ECR was inevitable, even if the details of timing and other characteristics may not have been predictable in advance.
Molecular clock evidence indicates that animals originated near 780 Ma, followed by the appearance of sponges, cnidarians, then by the time of the Marinoan glaciation (ending 635 Ma) the diversification of bilaterians (Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011; Erwin and Valentine Reference Erwin and Valentine2013). This pulse of diversification of bilaterian clades near the Ediacaran/Cambrian boundary documented by the fossil record is consistent with molecular evidence suggesting that most crown-group bilaterians date to the latest Ediacaran and Cambrian (Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011). The rapidity, phylogenetic breadth, and extensive morphologic disparity associated with the ECR has drawn forth a remarkable array of explanations from the reasonable and plausible to the patently absurd. Even among the more responsible hypotheses, however, there is a tension between explanations that invoke processes and mechanisms that are either occurring today or could plausibly be occurring today versus explanations that invoke unique circumstances and contingent events and are thus non-uniformitarian. This issue goes beyond the now predictable disputes between microevolutionists and taxic macroevolutionists over the nature of historical explanations of macroevolutionary patterns.
Here the question I address is similar to Vermeij’s, but with broader scope, if a narrower temporal focus. Few can dispute the transformative nature of the diversification of animals and attendant changes during the ECR, but did these unique events reflect unique circumstances, or merely unusual, historically contingent results of processes that have operated throughout the Phanerozoic? Specifically, I encompass a broader context than either Gould or Vermeij, including geological and geochemical changes to the environment during the early diversification of metazoans. More generally, what general conclusions about the nature of evolution can we draw from an understanding of the ECR? Paleontologists study speciation to derive general models of the speciation process. Similarly paleobiologists and other evolutionary biologists have explored larger-scale diversifications to identify recurrent macroevolutionary patterns (Jablonski Reference Jablonski2008, Reference Jablonski2010; Rabosky and Lovette Reference Rabosky and Lovette2008). Does the ECR provide general models of evolutionary innovation on this scale? I evaluate proposed causal factors in three domains: the physical environment, ecological interactions, and developmental processes. The purpose of this contribution is not to add to recent reviews of this topic (Budd Reference Budd2008; Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011) nor to evaluate competing hypotheses, but rather to inquire into the nature of differing explanations. Finally, I consider the implications of the tension between uniformitarian approaches and historically unique explanations both for understanding the ECR and for historical sciences more generally.
Environmental Context
Molecular clock evidence indicates that Metazoa originated during the Cryogenian (850–635 Ma) with sponges and cnidarians arising by about 700 Ma (throughout this contribution I use the dates from Erwin et al. [Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011], which are broadly consistent with other recent molecular clock studies of the metazoan radiation). The Cryogenian was bookended by two widespread glacial events: the Sturtian (ca. 720–660 Ma) and Marinoan (ca. 650–635 Ma), with a third, probably less extensive event punctuating the Ediacaran (the Gaskiers, ca. 580 Ma) (Halverson and Shields-Zhou Reference Halverson and Shields-Zhou2011). Some Sturtian and Marinoan glacial deposits were deposited in low paleolatitudes and are topped by lithologically distinctive carbonates known as “cap carbonates” (Shields Reference Shields2005). The high alkalinity and supersaturation of carbonate necessary to produce such a facies require highly unusual conditions, with the most extreme hypothesis being a Snowball Earth.
The Snowball Earth scenario involves glaciation near sea level extending to the equator, likely for millions of years (Hoffman et al. Reference Hoffman, Kaufman, Halverson and Schrag1998; Schrag et al. Reference Schrag, Berner, Hoffman and Halverson2002; Li et al. Reference Li, Evans and Halverson2013). The anomalous nature of a Snowball Earth extends to the structure of oceans underlying the ice. Modeling results suggest that extensive convective mixing would have produced vertically uniform temperature and salinity profiles (Ashkenazy et al. Reference Ashkenazy, Gildor, Losch, Macdonald, Schrag and Tziperman2013). Because release from the glaciation probably required sufficient buildup of atmospheric carbon dioxide from volcanic eruptions to overcome the reduction of chemical weathering of continents due to the buildup of ice, the deglaciation phase would have involved extreme environmental conditions, including high alkalinity and high nutrient loading of the oceans (Higgins and Schrag Reference Higgins and Schrag2003; Planavsky et al. Reference Planavsky, Rouxel, Bekker, Lalonde, Konhauser, Reinhard and Lyons2010). Although extensive glaciations occurred during the Phanerozoic, none were as widespread or had the geochemical impact of the Cryogenian events. Tziperman and colleagues (Tziperman et al. Reference Tziperman, Halevy, Johnston, Knoll and Schrag2011) proposed an intriguing scenario in which the glaciations were not triggered by physical processes, but rather were biologically mediated through increased transport of organic material to the deep ocean. Even if the Snowball Earth scenario is incorrect, the empirical evidence associated with the glaciations and their immediate aftermath, including the unusual cap carbonates, extreme isotopic changes, and other data, is sufficiently unusual as to require anomalous environmental conditions and a non-analog, and probably non-uniformitarian, explanation.
The amount of atmospheric oxygen increased about 2.4 billion years ago (Sessions et al. Reference Sessions, Doughty, Welander, Summons and Newman2009; Lyons et al. Reference Lyons, Reinhard and Planavsky2014) but Proterozoic oceans remained largely anoxic below a surface layer oxygenated by exchange with the atmosphere and local biological activity. These changes in ocean redox were likely also responsible for extensive changes in other geochemical proxies during the Cryogenian and Ediacaran including carbon, sulfur, and strontium isotopes as well as iron speciation (Halverson et al. Reference Halverson, Hurtgen, Porter and Collins2009, Reference Halverson, Wade, Hurtgen and Barovich2010; Maloof et al. Reference Maloof, Porter, Moore, Dudas, Bowring, Higgins, Fike and Eddy2010; Grotzinger et al. Reference Grotzinger, Fike and Fischer2011). Debate continues over the extent to which waters were euxinic (sulfur-rich), anoxic but not sulfidic (ferruginous), or anoxic but with low sulfate and ferruginous iron during the Neoproterozoic (Anbar and Knoll Reference Anbar and Knoll2002; Shen et al. Reference Shen, Canfield and Knoll2002; Canfield et al. Reference Canfield, Poulton, Knoll, Narbonne, Ross, Goldberg and Strauss2008; Johnston et al. Reference Johnston, Poulton, Dehler, Porter, Husson, Canfield and Knoll2010; Li et al. Reference Li, Love, Lyons, Fike, Sessions and Chu2010; Shields-Zhou and Och Reference Shields-Zhou and Och2011). Several recent papers have clarified the history of oxygen in the oceans during Neoproterozoic, with iron speciation and sulfur isotope data from the Fifteen-mile Group in the Yukon providing evidence of oxic shelfal waters overlying anoxic (largely Fe-rich but with occasional episodes of euxinia) deep waters after about 800 Ma (Sperling et al. Reference Sperling, Halverson, Knoll, Macdonald and Johnston2013b). Oxygen was increasingly present at levels sufficient for animals after the Marinoan glaciation, but shallow marine environments evidently lacked stable oxygen levels until about 560 Ma (Johnston et al. Reference Johnston, Poulton, Goldberg, Sergeev, Podkovyrov, Vorob’eva, Bekker and Knoll2012). The negative excursions in carbon isotopes, particularly the Shuram anomaly during the later Ediacaran Period, were more extreme than any yet documented for the Phanerozoic (Halverson et al. Reference Halverson, Wade, Hurtgen and Barovich2010; Grotzinger et al. Reference Grotzinger, Fike and Fischer2011; Lee et al. Reference Lee, Fike, Love, Sessions, Grotzinger, Summons and Fischer2013). Molybdenum and chromium isotopes also suggests pervasive ferruginous anoxia through the Proterozoic, with smaller regions of euxinic seafloor (Reinhard et al. Reference Reinhard, Planavsky, Robbins, Partin, Gill, Lalonde, Bekker, Konhauser and Lyons2013), and a recent model suggests that phosphorus levels may have been much lower than in modern oceans (Laakso and Schrag Reference Laakso and Schrag2014). The extreme carbon isotope perturbations documented during the late Neoproterozoic were progressively damped in the Early Cambrian, and although perturbations in stable isotopes and in other geochemical parameters occurred during some mass extinction events (e.g., Shen et al. Reference Shen, Crowley, Wang, Bowring, Erwin, Sadler, Cao, Rothman, Henderson, Ramezani, Zhang, Shen, Wang, Wang, Mu, Li, Tang, Liu, Liu, Zeng, Jiang and Jin2011), the system quickly reverted to the Phanerozoic norm. Although brief anoxic events occurred during the Paleozoic and Mesozoic, a state change seems to have occurred during the ECR to an oceanic redox state where pervasive and long-lasting anoxia was unlikely and perhaps impossible.
The critical question, however, is whether increased oxygen levels in shallow marine environments drove the ECR, and particularly the Cambrian Explosion, sensu stricto (Johnston et al. Reference Johnston, Poulton, Goldberg, Sergeev, Podkovyrov, Vorob’eva, Bekker and Knoll2012), or whether, as Butterfield has argued (Butterfield Reference Butterfield2009; Lenton et al. Reference Lenton, Boyle, Poulton, Shields-Zhou and Butterfield2014), there was sufficient oxygen in such settings far back into the Neoproterozoic. Recent geochemical evidence suggests that although the final ventilation of the oceans with oxygen may have occurred during the ECR, shallow-water settings had >1% present atmospheric levels of oxygen back as far as 800 Ma (Sperling et al. Reference Sperling, Halverson, Knoll, Macdonald and Johnston2013b). Moreover, Sperling et al. compiled data suggesting that the physiological oxygen requirements for bilaterian metazoans are much lower than many had previously assumed (see also similar data from Mills et al. [Reference Mills, Ward, Jones, Sweeten, Forth, Treusch and Canfield2014] for demosponges). Thus, despite the unusual environmental conditions of the late Neoproterozoic, lack of available oxygen does not seem to have prevented the early divergence of metazoans, but the Cambrian diversification of larger bilaterians (the Cambrian Explosion) likely did require the stable and higher oxygen levels found after 560 Ma.
An influx of nutrients associated with weathering or continental configuration has long been suggested as a possible explanation for the timing of the ECR (Valentine and Moores Reference Valentine and Moores1970; Brasier Reference Brasier1991). In their recent analysis of the “Great Unconformity” at the base of the Cambrian, Peters and Gaines (Reference Peters and Gaines2012) synthesized stratigraphic and geochemical data to identify anomalous patterns of sedimentation and, inferentially, seawater chemistry which may have been associated with patterns of biomineralization among early metazoans. The Great Unconformity has been recognized in many parts of the world, but using largely data from North America Peters and Gaines showed that it represents an interval of prolonged and extensive denudation and weathering, with the subsequent Early Cambrian transgression remobilizing weathering products and introducing them to the oceans. The unusual nature of this event is apparent in their data for the area of exposed basement rock, the burial flux of shelf carbonates, and the proportion of glauconite-bearing siliciclastic rocks, all of which were far higher in the Cambrian than at any other time in the Phanerozoic. Each of these indicators suggests a large flux of continental weathering products during the Cambrian, including ions important for biomineralization. The extensive production of glauconitic siliciclastic rocks in the inner detrital belt is best explained, according to Peters and Gaines, by this flux of weathering products; the chemical conditions associated with the formation of these deposits are much different from those found today. Consistent with this interpretation, the 87Sr/86Sr ratio climbed through the late Ediacaran and Cambrian, before reversing during the late Cambrian (Mazumdar and Strauss Reference Mazumdar and Strauss2006; Halverson et al. Reference Halverson, Wade, Hurtgen and Barovich2010). Concentrations of Ca2+ also increased substantially (Brennan et al. Reference Brennan, Lowenstein and Horita2004).
Thus a suite of singular and perhaps unprecedented environmental events occurred during the Cryogenian and Ediacaran: the global and low-latitude Sturtian and Marinoan glaciations, the extensive changes in ocean chemistry and the oxygenation of the deep oceans, and the pervasive continental weathering associated with the earliest Cambrian transgression. The available molecular clock data indicate that the early diversification of Metazoa occurred during this time. In addition, many other eukaryotic lineages diversified during the Neoproterozoic with a major radiation associated with the ECR (Knoll et al. Reference Knoll, Javaux, Hewitt and Cohen2006, Reference Knoll, Summons, Waldbauer and Zumberge2007). Although these environmental events are thus a necessary part of any explanation for the ECR, they are insufficient to explain the extent of the morphologic innovations (Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011; Erwin and Valentine Reference Erwin and Valentine2013). Glaciations, isotopic perturbations, redox changes, and unconformities have all occurred during the Phanerozoic and from a variety of causes, yet the magnitude of the environmental events during the late Neoproterozoic was unprecedented. It is obviously impossible to state that a similar suite of changes won’t recur, but many seem to reflect permanent state changes.
Ecological and Physiological Events
Redox and related Ediacaran and early Cambrian geochemical changes may have been aided, and possibly even driven, by biological innovations, further increasing the difficulties of unraveling the web of causal relations. In any case, some of these innovations forced state changes in ecological systems. Sponges evidently diversified during the Cryogenian, and on developmental and ecological grounds the last common metazoan ancestor was likely bactiverous with collar cells and a proto-epithelium. Although molecular clock studies suggest that many metazoan lineages predate 580 Ma, including crown-group cnidarians (and thus the cnidocyst) (Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011) we know little about their morphologies or the ecosystems in which they participated. Grazing mesoplankton and filter-feeding metazoans created the modern biological pump and helped ventilate the water column (Butterfield Reference Butterfield2009). By the second half of the Ediacaran, environments were dominated by microbial substrates and most Ediacara macrofossils were likely osmotrophic, feeding on dissolved organic carbon (DOC) (Laflamme et al. Reference Laflamme, Xiao and Kowalewski2009; Sperling et al. Reference Sperling, Peterson and Laflamme2011), a very limited trophic resource today. Macroscopic grazing habits appeared with Kimberella about 555 Ma (Fedonkin et al. Reference Fedonkin, Simonetta and Ivantsov2007). Metazoan trophic relations expanded enormously during the early stages of the Cambrian with filter-feeding, predation, and other trophic interactions. The advent of bilaterians with a through-gut increased bioturbation of marine sediments during the Cambrian, and reduced the distribution of microbial fabrics. This was not an abrupt transition, however, but a more gradual one with some Proterozoic-style microbial fabrics persisting into Cambrian Stage 5 (Dornbos et al. Reference Dornbos, Bottjer and Chen2005; Dornbos Reference Dornbos2006) as mixing of sediments slowly increased (Tarhan and Droser Reference Tarhan and Droser2014).
Three different classes of ecological interpretations of the ECR, and more specifically the diversification of bilaterian clades during the Early Cambrian (Stages 1–3), can be distinguished: (1) as an adaptive radiation; (2) as a recovery from previous mass extinction or environmental stress, with the focus on the influence of the Cryogenian glaciations and a putative end-Ediacaran mass extinction; and (3) associated with “key innovations” in multiple lineages triggering pervasive ecological changes, with primary attention to the onset of predation and burrowing.
Taking each of these possibilities in turn, the adaptive radiation view of the ECR has a long history (e.g., Stanley Reference Stanley1973; Conway Morris Reference Conway Morris1993; Schluter and McPhail Reference Schluter and McPhail1993). Here the metazoan diversification is seen as simply a more extensive (morphologically and phylogenetically) adaptive radiation, similar to those that have been well documented within many clades. The adaptive radiation view of the ECR assumes either that the fossil record provides a relatively reliable record of an Ediacaran diversification of either all Metazoa or the bilaterian clades (i.e., that there is not a hidden late Neoproterozoic history), or that a series of linked adaptive radiations occurred within each of the major clades participating in the ECR (and thus this view blends into the “key innovation” model). The ECR has few of the classic characteristics of an adaptive radiation: it occurred essentially simultaneously across numerous lineages and involved a much greater generation of morphologic disparity and taxonomic diversity (Erwin Reference Erwin1992; Erwin and Valentine Reference Erwin and Valentine2013). Describing the ECR as an adaptive radiation stretches the bounds of that term beyond recognition and has little explanatory value. A more useful approach would be to explore the mechanisms underlying broader-scale evolutionary radiations (Erwin Reference Erwin1992).
The Snowball Earth hypothesis and a postulated end-Ediacaran mass extinction have each been invoked as triggers for a post-crisis recovery. Despite the apparent severity of the Sturtian and Marinoan glaciations there are no data to suggest an evolutionary pulse following the amelioration of these conditions. The origin of eumetazoans and bilaterians roughly coincides with these two events, according to molecular clock estimates (Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011), but with the uncertainties on these estimates the origins could have come before the glaciations. The bilaterian divergences post-date the apparently less severe Gaskiers glaciation ca. 580 Ma, but this event is more directly connected to the appearance of rangeomorph and other components of the Avalon Ediacaran assemblage (Xiao and Laflamme Reference Xiao and Laflamme2008). Moreover, the molecular clock results of Erwin et al. (Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011) indicate that bilaterian crown group divergences are clustered in the latest Ediacaran and Cambrian. Although the Ediacaran fauna does disappear from the fossil record near the base of the Cambrian, there is no direct evidence for a mass extinction event at this horizon (Laflamme et al. Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013). Thus claims for a post-extinction evolutionary radiation are entirely speculative.
More plausibly, predation (Stanley Reference Stanley1973; Bengtson Reference Bengtson2002; Dzik Reference Dzik2007) and the development of vertical burrowing and bioturbation (McIlroy and Logan Reference McIlroy and Logan1999; Bottjer et al. Reference Bottjer, Hagadorn and Dornbos2000; Jensen et al. Reference Jensen, Droser and Gehling2005; Erwin and Valentine Reference Erwin and Valentine2013) have been seen as ecological innovations that could have triggered positive feedback leading to the widespread bilaterian diversification. Predation has long been a favored ecological explanation. Sperling et al. (Reference Sperling, Frieder, Raman, Girguis, Levin and Knoll2013a) compiled data on the ecology of polychaete assemblages in low-oxygen zones and showed that carnivores are absent in such settings. They linked the increase in marine oxygen during the late Ediacaran to the spread of carnivory, again coupling environmental and ecological explanations for the Cambrian radiation. The advent of active bioturbation is just as obviously linked to the Cambrian radiation. Bioturbation generates changes in redox gradients through the sediment, enhancing primary productivity and thus allowing increased biodiversity (Erwin and Tweedt Reference Erwin and Tweedt2011; Erwin and Valentine Reference Erwin and Valentine2013). The advent of bioturbation produced attendant changes in preservational style, and thus in the fossil record. Taphonomic settings of the Ediacaran with abundant microbial mats favored the preservation of soft tissues, in what Gehling described as a microbial “death-mask” model (Gehling Reference Gehling1999; see also Narbonne Reference Narbonne2005; Laflamme et al. Reference Laflamme, Schiffbauer, Narbonne and Briggs2010, Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013; Pawlowska et al. Reference Pawlowska, Butterfield and Brocks2013). With the onset of burrowing during the latest Ediacaran and early Cambrian these sedimentary fabrics were destroyed (Bottjer et al. Reference Bottjer, Hagadorn and Dornbos2000). The ecological changes associated with carnivory and burrowing were so fundamental, and so phylogenetically widespread, that they proved impossible to reverse later in the Phanerozoic. Both anoxic waters and mass extinctions drastically restricted the abundance of bioturbators, for example, but only for relatively short periods of time.
The morphologic disparity associated with the ECR is often described as one of the characteristic features, but I have not included it above, because it may not be a unique aspect of the ECR. In the first study of a diverse range of metazoan clades through the Phanerozoic, Hughes et al. (Reference Hughes, Gerber and Wills2013) show that maximal early disparity is characteristic of clades through the Phanerozoic (except for those whose range was truncated by one of the great mass extinctions). Thus, although the rapidity of the bilaterian diversification at the base of the Cambrian was unprecedented, as were the congruent increases in disparity across so many different clades, it is less clear that a major increase in disparity alone was a unique feature of this event.
Genetic and Developmental Networks
Comparative studies of development across extant metazoans have revealed patterns of deep homology among regulatory elements, including signaling pathways and transcription factors, as well as the processes of developmental evolution associated with the early history of metazoans leading to the ECR. Of particular significance for this discussion are (1) increases in the size of transcription factor families through gene duplication; (2) implications of the hierarchical structure of developmental gene regulatory networks (dGRNs), especially those involved in regional patterning of the developing embryo; and (3) increases in the complexity of microRNAs (miRNAs). Although gene duplication, including of transcription factors, and the growth of miRNAs persisted through the Phanerozoic, there were qualitative differences in the nature of some of the changes during early metazoan evolution that may have contributed to the unique events of the ECR. I should note at the outset that nothing described below involves mechanisms other than drift and selection. Rather, it is the effect that these processes have on the nature of subsequent genetic variation that differentiates them from other sorts of developmental changes.
Whole-genome sequencing has revealed that the basic set of bilaterian coding genes is 15,000–20,000, thus confirming that morphological disparity is a result of the regulatory patterning of these genes (Putnam et al. Reference Putnam, Srivastava, Hellsten, Dirks, Chapman, Salamov, Terry, Shapiro, Lindquist, Kapitonov, Jurka, Genikhovich, Grigoriev, Lucas, Steele, Finnerty, Technau, Martindale and Rokhsar2007; Carroll Reference Carroll2008; Erwin Reference Erwin2009; Simakov et al. Reference Simakov, Marletaz, Cho, Edsinger-Gonzales, Havlak, Hellsten, Kuo, Larsson, Lv, Arendt, Savage, Osoegawa, de Jong, Grimwood, Chapman, Shapiro, Aerts, Otillar, Terry, Boore, Grigoriev, Lindberg, Seaver, Weisblat, Putnam and Rokhsar2013). These networks of developmental control involve signal transduction (in which an extracellular signaling molecule activates a signaling pathway inside a cell, leading to expression of a transcription factor) and transcription factors (which generally lie close to a protein-coding gene and either activate or repress transcription of the gene, as with the canonical Hox genes). In addition to genes that produce regulatory proteins, RNA molecules may have regulatory functions of which the best studied in an evolutionary context are miRNAs. These short RNA molecules generally act as negative regulators on the expression of targeted genes, fine-tuning expression patterns and stabilizing development, particularly of cell types.
Whole-genome sequencing of choanoflagellates, sponges, cnidarians, and other basal metazoans provides a basis for estimating the complexity of the developmental genome in early animals. Three of the four most common eumetazoan signaling pathways—Wnt, Notch, and TGF-β—are present in sponges, as are elements of the fourth (Hedgehog) (Richards and Deganan Reference Richards and Deganan2009). Many transcription factors were also present in choanoflagellates and sponges but underwent expansion into families of related transcription factors before the origin of eumetazoans (Larroux et al. Reference Larroux, Luke, Koopman, Rokhsar, Shimeld and Degnan2008; Degnan et al. Reference Degnan, Vervoort, Larroux and Richards2009). For example, sponges have about 31 homeodomain transcription factors, which increased to about 61 in the cnidarian last common ancestor (LCA) and at least 82 for bilaterian LCA. The total number of classes of transcription factors (of which the homeobox class was just one) increased from 58 in sponges, to at least 87 in the cnidarian LCA and 115 in the bilaterian LCA (Larroux et al. Reference Larroux, Luke, Koopman, Rokhsar, Shimeld and Degnan2008). Because the increase in regulatory genes far outstrips the increase in protein-coding genes, this reinforces the point that a critical component of early metazoan evolution was the increased sophistication of the network of regulatory interactions.
These dGRNs have a semi-hierarchical structure of elements with varying evolutionary lability. The most downstream elements control protein-coding genes and evolve very rapidly. Far more refractory to evolutionary modification are genes associated with regional patterning of the developing embryo, for example involved in formation of the endomesoderm (gut), heart, etc. Davidson and his colleagues have intensively reconstructed the dGRN of the developing sea urchin embryo and identified a core of recursively wired regulatory genes that have been highly conserved over the past 500 million years (Davidson Reference Davidson2006; Hinman et al. Reference Hinman, Nguyen and Davidson2007; Davidson and Levine Reference Davidson and Levine2008; Hinman et al. Reference Hinman, Yankura and McCauley2009). These kernels are responsible for defining the spatial patterning for a particular region of the embryo. The five to six genes that compose this kernel are recursively wired and perturbation experiments have confirmed that disturbing any of them disables the entire patterning system (Davidson and Erwin Reference Davidson and Erwin2006, Reference Davidson and Erwin2010; Erwin and Davidson Reference Erwin and Davidson2009; Peter and Davidson Reference Peter and Davidson2011a). Comparative studies have shown that once these kernels formed they shifted evolutionary changes to upstream and downstream regions of the dGRN. The recursive wiring of the genes in the kernel means that multiple regulatory interactions are required in the control of any single gene. Furthermore, once formed, kernels appear to act as an evolutionary unit subject to selection, and thus the kernels define the limits to morphologic variation for that region of the embryo. The elucidation of kernels within the core of dGRN regional patterning appears, from the currently available data, to have occurred primarily during the early evolution of animals, and largely during the Ediacaran and possibly the Cambrian. Once formed, these kernels were enormously influential, but as with many major evolutionary innovations, they both created a design space and limited the scope and future possibilities of that space.
More recently, Peter and Davidson have constructed a Boolean model of the development process and compared the results with empirical data on gene expression pattern (Peter and Davidson Reference Peter and Davidson2011a,Reference Peter and Davidsonb). The comparative results show that the model of cis-regulatory interactions encompasses virtually all of the expression patterns in the developing embryo. This confirms that cis-regulatory interactions within the dGRNs are sufficient to account for developmental processes, without invoking regulatory interactions involve trans acting factors, extensive involvement of regulatory RNAs, or other factors.
The number of miRNAs is generally correlated with the morphological complexity of a clade. Major increases in miRNA complexity occurred between the cnidarian and bilaterian LCA, and again associated with the rise of vertebrates (Grimson et al. Reference Grimson, Srivastava, Fahey, Woodcroft, Chiang, King, Degnan, Rokhsar and Bartel2008; Wheeler et al. Reference Wheeler, Heimberg, Moy, Sperling, Holstein, Heber and Peterson2009; Christodoulou et al. Reference Christodoulou, Raible, Tomer, Simakov, Trachana, Klaus, Snyman, Hannon, Bork and Arendt2010). Once formed, most miRNAs seem to have been conserved. Losses of miRNAs are associated with clades that have experienced morphologic simplification, including flatworms, acoels, and Xenoturbella (Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011). Thus, to the extent that miRNAs have been involved in the generation of bilaterian morphologies, probably through stabilization of cell and tissue types, it appears that much of metazoan miRNA complexity was associated with the earliest phase of metazoan evolution, during the Cryogenian, and with the origin of vertebrates.
In summary, the global glaciations, changes in oceanic redox, the extent of perturbations to the carbon cycle documented by changes in carbon isotopes, and the extensive weathering have no parallels during the Phanerozoic and few apparent parallels earlier in earth history. The ecological changes involve the establishment of metazoan food webs, including predation and active burrowing. In the case of both the ecologic and the developmental changes, they are perhaps best characterized as encompassing the construction of the respective interaction networks. Subsequent evolutionary changes have been largely constrained to reorganization of these networks or the addition of new components.
Discussion
At some level of granularity any historical event is unique, and thus the question, “Was the Ediacaran–Cambrian radiation a unique evolutionary event?” seems a trivial and uninteresting one. Each speciation event, each biotic dispersal, and each trophic interaction is unique, but that does not prevent us from drawing general conclusions about the processes of speciation, dispersal, or ecological interaction. Historical sciences become more than narratives when they identify general patterns and regularities in mechanism from similarities among historically unique events.
In physics universal laws are described as symmetric because they are invariant in time and space: they are immune to change. Indeed the identification of symmetries, from Newton to Einstein to more recent physicists, has produced much of the power of modern physics. But some of the most intriguing issues in physics arise from phase transitions that break symmetries. The most famous and consequential of these are the transitions immediately after the Big Bang that led to the formation of the four fundamental physical forces. The equations operate the same across a symmetry-breaking event, but the physical nature of the particles, and the forces between them, has changed. And these changes have made all the difference.
Physicists describe systems where the dynamics are independent of initial conditions as ergodic and those where history matters and path-dependency is important as non-ergodic. The approaches and techniques needed to understand ergodic and non-ergodic systems are fundamentally different. Economists face the same dichotomy, although most of neo-Classical economic theory is fundamentally ergodic and ignores the path-dependent nature of economic change (Peters Reference Peters2011). As geologists we characterize this as a distinction between uniformitarian and non-uniformitarian processes (Gould Reference Gould1965), although this distinction does not completely capture the differences between ergodic and non-ergodic processes. Gould described the tension between idiographic or descriptive paleontological studies and nomothetic research (Gould Reference Gould1980) and proposed a hierarchical structure to historical processes in his “Paradox of the First Tier” discussion of the role of mass extinctions (Gould Reference Gould1985). Evolutionary biologists recognize the path-dependent and historical nature of evolutionary outcomes, but the structure of evolutionary theory, particularly population genetics, is invariant in time and space (Erwin Reference Erwin2011).
In Wonderful Life, Gould (Reference Gould1989) argued that contingency was the primary factor determining the long-term persistence of clades that arose during the Cambrian Explosion, particularly those revealed by the extraordinary preservation of the fossils of the Burgess Shale. Some evolutionary patterns, such as replicate adaptive radiations (Schluter and McPhail Reference Schluter and McPhail1993; Losos et al. Reference Losos, Jackman, Larson, de Queiroz and Rodriguez-Schettino1998; Mahler et al. Reference Mahler, Ingram, Revell and Losos2013) and convergences (Conway Morris Reference Conway Morris2009; Losos Reference Losos2011; McGhee Reference McGhee2011), strongly challenge the contingency of historical events by revealing an often unexpected degree of determinism in evolutionary patterns. Indeed the tension between contingency and determinism in evolution remains one of the more challenging issues in evolutionary theory. Ergodicity, uniformitarianism, and determinism are conceptually distinct, but each addresses the historicity (or lack thereof) in different domains.
Where does the ECR fit within the spectrum of contingency and determinism? And to what extent can one draw general lessons about evolutionary processes from events such as the ECR? Unlike Gould, whose interest lay in the aftermath of the ECR, here I am interested in the likelihood that an ECR-like event would have occurred if one “played the tape of life again,” including the environmental, developmental, and ecological circumstances. Specifically, if the extent of evolutionary innovation during the Ediacaran and early Cambrian was the result of a unique environmental and geochemical framework, or of transformational changes in ecosystem dynamics or the structure of ecological networks, then study of these processes may provide little insight for evolutionary theorists about general patterns of macroevolutionary change. Alternatively, one could argue that despite the unique aspects of the Ediacaran–Cambrian interval chronicled above, the end result, the ECR, would have played out in a very similar way.
Nature of Determinism and Contingency
When Gould used the term contingency and the metaphor “replaying the tape of life,” he was not explicit in his definitions of the term (Beatty Reference Beatty2006). In response to the claims made by Gould in Wonderful Life philosophers of science have distinguished five different senses of contingency (for a more extensive discussion, see Reference ErwinErwin in press): (1) Sampling error, which Gould explicitly rejected as a form of contingency but which may have greater application to the phenomenon than he realized. (2) Unpredictability of the course of history. (3) Causal dependency on, or sensitivity to, initial conditions (Beatty Reference Beatty2006). Sensitivity to initial conditions (hereafter SIC) is often found in chaotic dynamics, and it contrasts with systems in which neither the initial starting conditions nor the history of the system influences the final result. Such systems are often described as having a basin of attraction. Beatty suggested that although contingency as described by (2) and (3) can be complementary, Gould did not distinguish between them and often conflated them. Beatty suggested that Wonderful Life could be interpreted as supporting either the unpredictability of history or sensitive dependence on initial conditions. (4) Sensitivity to external disturbance, which is related to system resilience (Inkpen and Turner Reference Inkpen and Turner2012). This sense of contingency seems particularly applicable to mass extinctions and similar, externally triggered events. And finally, (5) Macroevolutionary stochasticity, the unbiased sorting among species over macroevolutionary time, which Turner (Reference Turner2011) argues best encapsulates the sense in which Gould used the term contingency. (Note that I have avoided the term “path-dependence” in this paper, a term that has often been applied in the sense of sensitivity to initial conditions in historical settings, but is also used as simply implying a historical process, and thus can be imprecise.) An interesting extension of these ideas was presented by Inkpen and Turner (Reference Inkpen and Turner2012) who proposed that the “topography” of historical contingency may itself change over time. Such circumstances produce a situation where B is not inevitable, but should A occur, B is almost certain to occur; Sterelny (Reference Sterelny2005) described this as conditional inevitability.
Sensitivity to initial conditions and unpredictability provide an initial framework for delineating domains of contingency and determinism (Table 1). If a system is both subject to SIC and unpredictable (domain 1), then it is fully contingent in both senses meant by Beatty, and the history of the system would be highly important to the outcome of events. However, the extensive historicity would make generalizing across cases difficult. Our modern view of human history falls into this category. In domain 2, the system is still subject to SIC, but the evolutionary pathways of lineages are predictable or deterministic. As in domain 1, the SIC would mean that different “runs” of the history of life would have little similarity, but within runs evolutionary patterns would generate parallelisms and convergence. This is the domain where historical contingency changes over time, as described by Inkpen and Turner (Reference Inkpen and Turner2012) and encompasses Sterelny’s (Reference Sterelny2005) “conditional inevitability.” The opposite situation arises in domain 3, where there is no SIC but historical trajectories are unpredictable. Turner’s (2012) macroevolutionary stochasticity would be one example of such a dynamic. Domain 4 encompasses systems in which neither SIC nor unpredictability is important and the outcome is deterministic. For both domains 3 and 4, the absence of SIC indicates the presence of basins of attraction so that repeated runs would generate the same or similar results.
Table 1 Domains of sensitivity to initial conditions and unpredictability.
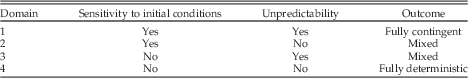
If I understand the arguments of Conway Morris (Reference Conway Morris2009) correctly, he argues that domains 3 and 4 dominate the history of life. General themes are essentially inevitable, generating convergence, even if the specific instantiations of them are not. I believe (although I am not sure) that Vermeij’s (Reference Vermeij2006) arguments about repeated innovation also fall within these domains. At the opposite extreme from domain 1 is the fully deterministic situation of domain 4 where SIC is absent and the evolutionary pathways of lineages are predictable or deterministic. This is the domain of laws, where replaying the tape of life would generate similar outcomes and convergence over alternative runs would be high (likely subject only to stochastic fluctuations). In such a system history would be of little importance because replicate runs would produce largely the same result. It should be of little surprise that this is the domain of much of physics and the realm where many economists imagine that they live.
Applications to the Ediacaran–Cambrian Radiation
The evidence summarized here suggests that the unique nature of the ECR extends beyond the suddenness and morphologic breadth of evolutionary innovation, particularly at the base of the Cambrian. But the unique nature of these events does not necessarily help in understanding the extent of contingency and determinism with respect to the ECR. We can use the four domains of Table 1 to identify the attributions of different components of the ECR. As always with discussions of contingency and determinism, carefully specifying the focal level of interaction is critical.
Most of the environmental aspects of the ECR, particularly the perturbations to the carbon cycle and the increased weathering associated with the Great Unconformity, fall within domain 1, although the latter probably came too late within the ECR to have substantially affected the evolutionary trajectory. If the glaciations were caused by continental positions and drawdown of carbon dioxide, as may have been the case, then they might be better placed in domain 2 or even 4. The situation with oxygen is more complicated. Geologic evidence now strongly indicates that by 800 Ma oxygen in shallow waters was sufficient for the origin of animals (Sperling et al. Reference Sperling, Frieder, Raman, Girguis, Levin and Knoll2013a,Reference Sperling, Halverson, Knoll, Macdonald and Johnstonb). Thus one could argue that the oxygenation of shallow waters falls within domain 2, conditionally inevitable after the advent of oxygenic photosynthesis (even if the timing was less constrained). However, although there were small amounts of oxygen present it was likely insufficient to permit the larger metazoan body sizes that appeared during the ECR, which required a substantial increase in oxygen. Several workers have now suggested that the ventilation of the oceans was driven largely by biological processes (Butterfield Reference Butterfield2009; Erwin and Valentine Reference Erwin and Valentine2013; Lenton et al. Reference Lenton, Boyle, Poulton, Shields-Zhou and Butterfield2014; Mills et al. Reference Mills, Ward, Jones, Sweeten, Forth, Treusch and Canfield2014). Such a biotically driven process would be unpredictable but the relationship to SIC is unclear at present; thus this would fall into either domain 1 or domain 3.
As with the development of increased oxygen levels, one can distinguish two different phases of unique developmental innovations: the origin of the metazoan developmental toolkit, with significant input from developmental processes found among unicellular eukaryotes, and the subsequent expansion of that toolkit associated with increased morphologic complexity and the development of more complex morphogenetic pathways. The first of these phases seems to fall firmly within domain 1, as evidenced by the fact that only a single lineage (leading to sponges) made this transition. In contrast, the expansion of this toolkit to generate developmental GRNs and pathways occurred multiple times, independently, in different clades. Appendages, guts, eyes and other aspects of bilaterian body plans are generally unique to specific clades, even when the underlying developmental machinery shares highly conserved genes and patterns of gene interaction. Thus these events appear to fall within domains 2 and 4: The hierarchical patterns of regulatory interaction may be an inherent outcome of the increased sophistication of regulatory networks, and thus fall within domain 4, whereas other aspects of the morphogenetic pathways may have been more sensitive to initial conditions and thus fall within domain 2.
Finally, among the unique ecological components of the ECR, most basic trophic interactions, including predation, detrivory, and others occur within microbial assemblages. Although the appearance of some trophic interactions, such as predation, were associated with the Cambrian radiation (Erwin et al. Reference Erwin, LaFlamme, Tweedt, Sperling, Pisani and Peterson2011; Sperling et al. Reference Sperling, Frieder, Raman, Girguis, Levin and Knoll2013a), the ubiquity of such interactions suggests that their appearance is neither unpredictable in the sense of this paper, nor subject to SIC. Consequently these interactions fall within domain 4.
To the extent that these assignments of different aspects of the early origins of metazoans to different domains are accurate, this suggests that the unique components of the ECR fall across a spectrum from highly contingent to highly deterministic. Most of the environmental events appear to have been both unpredictable and have high SIC. At the opposite extreme of low historicity and high determinism (domain 4) lies the growth of ecological and developmental interaction networks. Domain 2 of conditional inevitability includes some aspects of the increased oxygenation of marine waters and perhaps the expansion of morphogenetic pathways of development. Molecular clock evidence indicates that the origin of most major metazoan clades during the Cryogenian and early Ediacaran, and the concomitant establishment of most elements of the metazoan developmental toolkit, happened 100–150 Myr before the Cambrian Explosion.
In the preceding discussion I assumed the absence of feedback and thus that evolutionary lineages were passively responding to environmental pressures but lacked the ability to actively modify them. But feedback processes do exist through which organisms modify their own environment, and these have recently received extensive treatment as niche construction (Odling-Smee et al. Reference Odling-Smee, Laland and Feldman2003; Laland and Sterelny Reference Laland and Sterelny2006) and ecosystem engineering (Jones et al. Reference Jones, Lawton and Shachak1997; Cuddington et al. Reference Cuddington, Byers, Wilson and Hastings2007), including their importance over macroevolutionary time scales (Erwin Reference Erwin2008; Erwin and Tweedt Reference Erwin and Tweedt2011). During the ECR, the appearance of widespread filter-feeding by sponges in the Ediacaran, the sequestration of carbon due to production of fecal pellets by pelagic bilaterians (Mills et al. Reference Mills, Ward, Jones, Sweeten, Forth, Treusch and Canfield2014) and the onset of burrowing activities in the earliest Cambrian each had the potential to significantly change redox of the oceans and shallow marine sediments (Erwin and Valentine Reference Erwin and Valentine2013). Although the effect of such changes has yet to be rigorously established, this provides an example where an evolutionary innovation could feed back to affect the probabilities of other changes. This could have enhanced the probability of successful evolutionary changes dependent upon oxygen availability (including increased muscles, body size, and active predation). The feedback associated with ecosystem-engineering activities could push some of the ecological interactions into domain 3. While this paper was in review Doebeli and Ispolatov (Reference Doebeli and Ispolatov2014) discussed the dynamics of nonlinear feedbacks associated with frequency-dependent selection. Their analysis suggests that in many cases chaotic dynamics are expected rather than predictable outcomes. Addressing the effect of feedbacks on the dynamics of the ECR is an issue worthy of further study.
Although some aspects of the ECR appear to have involved a significant degree of determinism, and thus are likely repeatable under the appropriate conditions, other aspects of this episode were highly contingent. The rapid burst of morphological innovation among bilaterian clades during the latest Ediacaran and earliest Cambrian, which is the focus of considerable interest, largely involved three factors: an increase in oxygen levels, although the cause is unclear, and a growth in developmental and ecological interaction networks. These events were probably conditionally inevitable once Metazoa had originated and undergone their initial diversification. As discussed above, these earlier events appear to have involved a greater degree of contingency.
Implications for Evolutionary Theory
In his paper on “The paradox of the first tier” Gould (Reference Gould1985) proposed that evolution was hierarchically and discontinuously structured, progressing along distinct tiers: the ecological dynamics of the first tier, the evolutionary trends among lineages and clades of the second tier, and the dynamics of mass extinctions in the third tier. The argument developed here expands Gould’s argument to the dynamics of evolutionary radiations, proposing that the events of the ECR were both quantitatively different and qualitatively distinct from other evolutionary events. The conditional inevitability of the bilaterian expansion and the contingent nature of early events in metazoan evolution suggest that although the ECR provides critical information about the dynamics of evolutionary innovation and the diversity of evolutionary events in the history of life, it may be much less informative about more frequent aspects of macroevolutionary change.
The role of conditional inevitability (Sterelny Reference Sterelny2005) or the topology of historical contingency (Inkpen and Turner Reference Inkpen and Turner2012) is an issue worth further investigation. Even the preliminary discussion has identified a number of areas in which the roles of contingency and determinism have changed over time, with the role of contingency often becoming increasingly constrained. Traditional views of both macroevolution and microevolution have been largely uniformitarian (Erwin Reference Erwin2011), and greater attention to the dynamics of historical contingency might generate a more historical informed view of evolutionary possibility.
The Ediacaran and Cambrian was not the only interval of unidirectional changes in the Earth system or of feedbacks between biological evolution and changes in the physical environment. For example, the spread of vascular plants changed terrestrial weathering patterns and shifted rivers from sheet-braided to meandering channels with abundant mud and clay (Gibling and Davies Reference Gibling and Davies2012). Gibling and Davies argue that the development of terrestrial plant ecosystems beginning in the Silurian coupled the evolution of landscapes to evolutionary changes in both plants and animals, with plants acting as “geomorphic engineers,” altering fluvial landscapes and thus their own evolution.
An example in which the dynamics of the systems appear to have been quite similar even though the initial conditions and external perturbations were very different is the end-Permian versus the end-Cretaceous mass extinction. Two very different settings, and with very different causes: massive volcanism in the case of the end-Permian (Shen et al. Reference Shen, Crowley, Wang, Bowring, Erwin, Sadler, Cao, Rothman, Henderson, Ramezani, Zhang, Shen, Wang, Wang, Mu, Li, Tang, Liu, Liu, Zeng, Jiang and Jin2011; Burgess et al. Reference Burgess, Bowring and Shen2014) and the impact of an extra-terrestrial bolide at the end of the Cretaceous (Schulte et al. Reference Schulte, Alegret, Arenillas, Arz, Barton, Bown, Bralower, Christeson, Claeys, Cockell, Collins, Deutsch, Goldin, Goto, Grajales-Nishimura, Grieve, Gulick, Johnson, Kiessling, Koeberl, Kring, MacLeod, Matsui, Melosh, Montanari, Morgan, Neal, Nichols, Norris, Pierazzo, Ravizza, Rebolledo-Vieyra, Reimold, Robin, Salge, Speijer, Sweet, Urrutia-Fucugauchi, Vajda, Whalen and Willumsen2010). Despite these differences, one of the most striking features of the two events is the similarity in the rate and pattern of biotic collapse (Erwin Reference Erwin2006). This suggests, for reasons that I think remain unclear, that the collapse of Earth’s ecosystems during such crises follows very similar trajectories independent of the actual forcing factors (Erwin Reference Erwin2014).
Acknowledgments
I appreciate discussion of these topics with E. Davidson, C. Haufe, A. Love, and J. W. Valentine, none of who are responsible for the views expressed here. I appreciate comments on an earlier draft of the manuscript from S. H. Xiao and an anonymous reviewer. This research was originally presented at the 2012 Geological Society of America meeting at a session organized by J. Schiffbauer and S. H. Xiao, to whom I am indebted for the invitation to speak. This research was funded by the NASA National Astrobiology Institute through the MIT node.



