This paper contains supplementary material that can be found online at http://journals.cambridge.org
Introduction
The Mediterranean Sea, home to many threatened and endemic species, has suffered a general decrease in biodiversity, with temporal trends indicating that overexploitation and habitat loss have been the main drivers of this change and that these influences are expected to increase (Coll et al., Reference Coll, Piroddi, Steenbeek, Kaschner, Ben Rais Lasram and Aguzzi2010). Overexploitation on rocky shores involves people collecting animals and plants for food or bait (Keough et al., Reference Keough, Quinn and King1993); on the accessible intertidal areas there are many target species, such as limpets, that are extensively collected (Lindberg et al., Reference Lindberg, Estes and Warheit1998). An example is Patella ferruginea, the most threatened marine macroinvertebrate in the western Mediterranean (Ramos, Reference Ramos1998), which is included in several directives at European and country levels (MMAMRM, 2008). This species has been collected since the Pleistocene (Espinosa et al., Reference Espinosa, Rivera-Ingraham, Fa and García-Gómez2009a). Exploitation can decrease the reproductive output of intertidal invertebrate populations because individual fecundity of many species increases with body size (Levitan, Reference Levitan1991; Tegner et al., Reference Tegner, Basch and Dayton1996).
According to MMAMRM (2008) P. ferruginea occurs in North Africa (e.g. Ceuta, Melilla); the Chafarinas Islands (Spain); Al Hoceima Natural Park, Morocco; the Rachgoun and Habibas Islands (Algeria); the Cap Bon Peninsula and Zembra Island (Tunisia); and the southern Iberian Mediterranean coast, Corsica and Sardinia. However, there is little known about the status of these populations and most of the available information dates from >25 years ago. The only research addressing the global distribution of P. ferruginea was that of Laborel-Deguen & Laborel (Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991a), although much of the data provided by them were from palaeological sources rather than direct census. It has been suggested that the increase in artificial structures in the Mediterranean could lead to a general loss of genetic biodiversity, populations and species (Fauvelot et al., Reference Fauvelot, Bertozzi, Constantini, Airoldi and Abbiati2009; Bulleri & Chapman, Reference Bulleri and Chapman2010). Nevertheless, several species, including P. ferruginea, have settled on artificial substrates, where dense populations can be found (García-Gómez et al., Reference García-Gómez, López-Fé, Espinosa, Guerra-García and Rivera-Ingraham2011). However, there is a lack of information about how the type of substrate influences the population dynamics of this species. The aim of this research was to provide updated information for the entire western Mediterranean, to evaluate the current status of this species. Additionally, we tested the hypothesis that population structure and related reproductive success can be influenced by collection by people and by the type of substrate on which the populations are settled (artificial vs natural). The results will assist in the conservation management of this species.
Study area
We explored the shores of the mainland and of Zembra and Zembretta islands, Tunisia, Pantelleria and the Egadi islands off western Sicily, the western Sicilian mainland, Corsica, Sardinia and the Tuscany–Liguria coast of Italy (Fig. 1, Supplementary Table S1). We visited sites where the species had been recorded previously on the Egadi islands and Pantelleria (Giaccone & Sortino, Reference Giaccone and Sortino1974), along the Tuscany–Liguria coast (La Rochette: Curini-Galletti, Reference Curini-Galletti1979; Quercianella: Terreni, Reference Terreni1981; Piombino: Biagi & Poli, Reference Biagi and Poli1986; Portofino: Porcheddu & Milella, Reference Porcheddu, Milella, Boudouresque, Avon and Gravez1991) and on Corsica (Laborel-Deguen & Laborel, Reference Laborel-Deguen and Laborel1990, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991b). We also collected all available information on the status of the species throughout the Mediterranean.
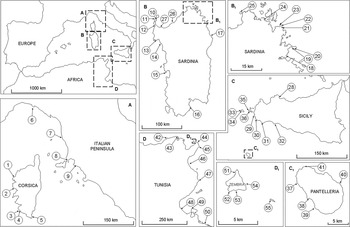
Fig. 1 Locations of the 55 study sites where we surveyed Patella ferruginea. For details of each site see Supplementary Table S1.
Methods
Sampling sites were selected on rocky habitats, where the species settles, mainly in marine protected areas or at locations with healthy marine communities (because of the sensitivity of the species to pollution and collection; Espinosa et al., Reference Espinosa, Guerra-García and García-Gómez2007). Transects parallel to the coast were surveyed at each locality, by snorkelling on steep or sloping shores and on foot on shallow sloping shores. All limpets settled on the shore were recorded. At localities with sparse populations 100 m transects were surveyed, whereas 10 m transects were used at sites with high densities (Supplementary Table S1; Laborel-Deguen & Laborel, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991b; Guerra-García et al., Reference Guerra-García, Corzo, Espinosa and García-Gómez2004). All specimens of P. ferruginea found were measured with a calliper to the nearest mm along the longitudinal axis (Guerra-García et al., Reference Guerra-García, Corzo, Espinosa and García-Gómez2004). Small individuals are difficult to detect (Guallart et al., Reference Guallart, Templado, Calvo, Cabezas, Acevedo, Machordom and Luque2006) and therefore particular care was taken to record this component of the population. For graphical representations of size frequencies we have only taken into consideration sites with at least 10 specimens. Data from the sites surveyed in the Zembra archipelago were pooled because genetic analyses have shown that there is no limitation to gene flow in this area (Casu et al., Reference Casu, Rivera-Ingraham, Cossu, Lai, Sanna and Dedola2012).
The assumptions for parametric statistics were not met by some of our data, with severe departures from normality and highly unequal variances. The ANOVA test is, however, robust for deviation from normality (Underwood, Reference Underwood1997) and if a Type I error is minimized (by using P<0.01) it is possible to conduct an analysis even if the condition of equal variances is not met. Differences between means were examined a posteriori using the Student–Newman–Keuls test. Multivariate MDS (non-metric multidimensional scaling) statistics were also used, based on the UPGMA method (unweighted pair-group method using arithmetic averages), along with the Bray–Curtis similarity index. MDS was used to test differences in population structure among sites (i.e. number of specimens in each 1-cm size class from 0 to 10 cm). Clusters of sites identified as statistically significant using the profile test SIMPROF (P < 0.05) were considered to have a similar population structure. Kruskal's stress coefficient was used to test the ordination (Kruskal & Wish, Reference Kruskal and Wish1978). PERMANOVA (permutational multivariate analysis of variance) was used to test hypotheses regarding differences in population structure between types of substrate (natural vs artificial) and accessibility, assuming that at less accessible sites the limpets are more protected from collection (protected vs unprotected). Analyses were performed using a log(x+1) transformation for the MDS analysis and fourth-root transformation for the PERMANOVA test, to lessen the influence of the more abundant size classes. The abundance of each size class was standardized to density (individuals per m of transect) to avoid artefactual differences among populations resulting from the differing sampling effort at each site. Data for the MDS and PERMANOVA analyses were compiled from the present study, augmented by information from Granada and Alboran island (Spain; Barba et al., Reference Barba, Nevado, Moreno, De La Linde, Remón and De La Rosa2006), Crinavis (Algeciras Bay, Spain; Navarro-Barranco, Reference Navarro-Barranco2010), San Felipe breakwater (Algeciras Bay, Spain; Espinosa et al., Reference Espinosa, Fa and Ocaña2005), Ceuta (North Africa, Spain; Espinosa et al., Reference Espinosa, Rivera-Ingraham, Fa and García-Gómez2009a), Habibas and Plane islands (Algeria; Espinosa, Reference Espinosa2009) and Melilla (North Africa, Spain; González-García et al., Reference González-García, Bueno Del Campo, García-Piña and Bazairi2006). Univariate and multivariate analyses were carried out using SPSS v. 15.0 (SPSS, Chicago, USA) and PRIMER v. 6.0 (Clarke & Gorley, Reference Clarke and Gorley2006).
Results
In total 1,063 individuals of P. ferruginea, distributed non-homogeneously (Supplementary Table S1; Table 1; Fig. 2), were recorded and measured. The species is extremely scarce on mainland Tunisia (Fig. 2e,f; in the Cap Bon area, although there are other small populations from the Gulf of Tunis to Monastir, see Discussion). A large and well-structured population was recorded in the Zembra archipelago (mean density 2.65 m−1), with high levels of recruitment and large, reproductively mature specimens (Fig. 2b). On the Egadi islands we found four individuals (4.0, 4.0, 5.2, 6.5 cm in length) at Punta Mugnona, three at Grotta Preseppe (5.0, 5.5, 6.5 cm; both sites on Marettimo Island), and three at Punta Sottile (2.1, 3.0, 4.6 cm; Favignana Island). Only one individual (1.9 cm) was recorded on Pantelleria, at Punta Rosso di Nica. On Corsica and Sardinia the populations were highly fragmented and densities were low, with only one individual (2.4 cm) found at Bonifacio and four at Tizzano (1.6, 2.2, 2.5, 5.6 cm), although some sites, such as Asinara (Sardinia), had more individuals (Fig. 2a). The species was absent from mainland Italy and Sicily. There were significant differences in mean shell length among populations, with the largest individuals at Asinara (Table 2).
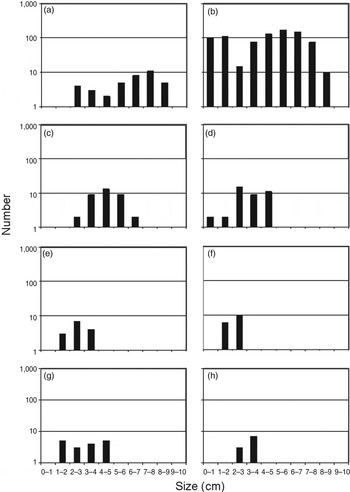
Fig. 2 Size frequency distribution of P. ferruginea (on a logarithmic scale) for each site at which >10 specimens were collected (Supplementary Table S1): (a) Asinara (Site 10), (b) Zembra archipelago (data pooled for Sites 51–55), (c) Capo Testa (25), (d) Galeria (1), (e) Kelibia (45), (f) El Haouaria (44), (g) Porto Rafael (24), (h) Olbia (18).
Table 1 Summary statistics for the size distribution of Patella ferruginea (only for sites where the species was detected). Site numbers (Supplementary Table S1; Fig. 1) are in parentheses.
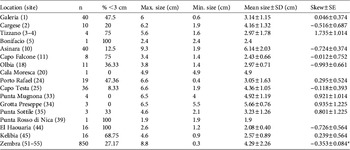
* Skew is considered significant when its value is >2 x SE.
Table 2 One-way ANOVA for the differences in size between populations of P. ferruginea. Only the eight populations where >10 individuals were recorded were included in this analysis. Results of the Student–Newman–Keuls test are ordered by mean size from high to low.
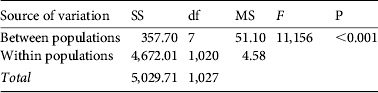
Student–Newman–Keuls test: Asinara>Capo Testa = Zembra = Galeria = Porto Rafael = Olbia = Kelibia = El Haouaria.
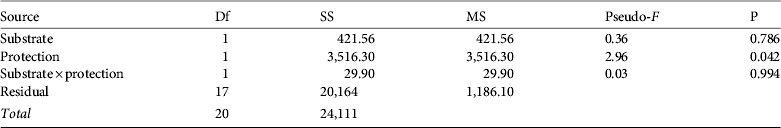
Multivariate analyses indicated differences in population structure with the level of exploitation. Populations in areas free of human visitation (protected) partially segregate from the human-affected sites in the MDS analysis, mainly appearing in the upper half of the graph (Fig. 3). SIMPROF analysis showed three clusters: one of small populations at frequently visited sites (less protected) on Corsica, Sardinia and mainland Tunisia, a second of mainly protected sites (both on natural and artificial substrates), and a third of several North African populations from the Strait of Gibraltar to the Zembra archipelago in the Siculo-Tunisian strait. PERMANOVA analysis indicated that protection (as a result of inaccessibility) influenced the size frequency distribution of the populations, whereas the type of substrate (natural vs artificial) had no significant influence on population structure (Table 3).

Fig. 3 MDS ordination of populations (only those with >10 individuals) of P. ferruginea based on the number of individuals per size class. Groups of samples determined by the SIMPROF test are enclosed by ellipses. Populations include six from this study (Table 2) and 15 from published data (Table 4). Designation as unprotected (circles) or protected (triangles) indicates the likelihood or not, respectively, of exploitation, based on accessibility. ANP, artificial substrate, non-protected; AP, artificial substrate, protected; NNP, natural substrate, non-protected; NP, natural substrate, protected. Arrows indicate artificial substrates.
Table 3 PERMANOVA analysis of the effect of substrate and protection on the size (measured on the longitudinal axis) structure of P. ferruginea populations. The matrix used was the same as for the MDS analysis (Fig. 3).
Discussion
The main conclusion of this study is the vulnerable situation of P. ferruginea. Although previous studies identified the threatened status of the species (Paracuellos et al., Reference Paracuellos, Nevado, Moreno, Giménez and Alesina2003; Bazairi et al., Reference Bazairi, Salvati, Benhissoune, Tunesi, Rais and Agnesi2004; Guerra-García et al., Reference Guerra-García, Corzo, Espinosa and García-Gómez2004; Espinosa, Reference Espinosa2006; Cristo & Caronni, Reference Cristo and Caronni2008; Espinosa et al., Reference Espinosa, Rivera-Ingraham, Fa and García-Gómez2009a,Reference Espinosa, Maestre and García-Gómezb, and references therein), mainly a result of human pressure, these studies were at a local or regional scale. The information summarized here demonstrates that the range of this threatened mollusc has contracted severely and that the remaining populations are scarce and generally sparse.
The species has not been reported from the Italian mainland since the 1980s, when it was recorded in low numbers at a few locations in Tuscany (Curini-Galletti, Reference Curini-Galletti1979; Terreni, Reference Terreni1981; Biagi & Poli, Reference Biagi and Poli1986) and one location in Liguria (Porcheddu & Milella, Reference Porcheddu, Milella, Boudouresque, Avon and Gravez1991). However, we did not find the species at any of these locations. The situation on the Italian islands of Egadi, Sicily and Pantelleria is also critical. Giaccone & Sortino (Reference Giaccone and Sortino1974) reported P. ferruginea on the Egadi islands with a relative abundance of II, within a range from I (rare) to V (abundant), but it is now extremely scarce, having also declined in density. Giaccone et al. (Reference Giaccone, Sortino, Solazzi and Tolomio1973) reported the species in a general phytobenthos study at Pantelleria but we detected only one small specimen there, despite the focus on this species. There are some specimens from Palermo, Sicily, in Locard's 1892 collection at the Muséum national d'Histoire naturelle, Paris, but the species no longer occurs on the island.
The Corsican and Sardinian populations were greater in density than those on the Egadi islands and Pantelleria but there has also been a noticeable decline on these islands, with a large decrease in mean density at several sites from 1985 to 2009: 0.933 to 0.02 m−1 at Tizzano (Laborel-Deguen & Laborel, Reference Laborel-Deguen and Laborel1990; present study) and 2.334 to 0.13 m−1 at Gáleria (Laborel-Deguen & Laborel, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991b; present study; Fig. 4). Exploitation in Corsica during summer has been reported by Laborel-Deguen & Laborel (Reference Laborel-Deguen and Laborel1990, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991b).

Fig. 4 Size (measured on the longitudinal axis) frequency distribution of P. ferruginea at Galéria, Corsica, in (a) 1985 (data from Laborel-Deguen & Laborel, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991b) and (b) 2009 (present study).
In Tunisia P. ferruginea is restricted to the north-eastern shores, as also reported by Tlig-Zouari et al. (Reference Tlig-Zouari, Rabaoui, Hosni and Ben Hassine2010). The largest and most structured population (i.e. presence of different size classes) is on the Zembra archipelago, although Tlig-Zouari et al. (Reference Tlig-Zouari, Rabaoui, Hosni and Ben Hassine2010) reported lower densities at Zembra than at Cap Bon. However, on Zembra they surveyed at a location with the lowest densities, which is probably not representative of the archipelago. The species shows great variability in recruitment (Guallart et al., Reference Guallart, Templado, Calvo, Cabezas, Acevedo, Machordom and Luque2006; Rivera-Ingraham, Reference Rivera-Ingraham2010) and the high densities found at some sites at Cap Bon by Tlig-Zouari et al. (Reference Tlig-Zouari, Rabaoui, Hosni and Ben Hassine2010) could be a result of good recruitment from the Zembra population during 2006.
Many authors have demonstrated the influence of exploitation on the mean size and presence of large reproductive individual molluscs (Keough et al., Reference Keough, Quinn and King1993; Branch & Odendaal, Reference Branch and Odendaal2003). Patella ferruginea is used as food and fishing bait and as a collector's item (Laborel-Deguen & Laborel, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991a, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravezb; Templado & Moreno, Reference Templado and Moreno1997; Ramos, Reference Ramos1998), the latter confirmed by our observations of shells being sold as souvenirs at the tourist site of Kerkouan, Carthage. Accessibility is the most important factor influencing collection, regardless of the legally protected status of the species (Guerra-García et al., Reference Guerra-García, Corzo, Espinosa and García-Gómez2004; Espinosa et al., Reference Espinosa, Rivera-Ingraham, Fa and García-Gómez2009a). To protect threatened molluscs such as P. ferruginea we recommend, based on our findings, use of marine protected areas combined with effective control of human visitation, as has been implemented on Asinara island and the Zembra archipelago. Although exploitation is evident in several areas of Sardinia (Cristo & Caronni, Reference Cristo and Caronni2008), the marine protected area of Asinara has the largest individuals and highest densities in the Corsica–Sardinia region, and the population in the Zembra marine protected area (established in 1977 and where human visitation is prohibited) showed strong recruitment and had many large reproductive individuals, indicating a well-structured and viable population (Espinosa et al., Reference Espinosa, Rivera-Ingraham, Fa and García-Gómez2009a). Changes in the population on Zembra during the last 25 years have been positive (Fig. 5) in terms of density (from 0.7 m−1 in 1986 to 2.65 m−1 in 2009) and mean length (mean of specimens >2 cm was 4.4 cm in 1986 and 5.4 cm in 2009). Marine protected areas (Asinara, Zembra: present study; Habibas and Chafarinas islands: Espinosa, Reference Espinosa2009; Guallart et al., Reference Guallart, Templado, Calvo, Cabezas, Acevedo, Machordom and Luque2006) and also non-legally protected sites, many of them on artificial substrates (e.g. Algeciras Bay and Ceuta; Espinosa et al., Reference Espinosa, Rivera-Ingraham, Fa and García-Gómez2009a), could be a source of larvae for adjacent areas. Connectivity between populations needs to be guaranteed by establishment of a large number of small and proximate (no more than 10–20 km apart) marine protected areas, as suggested by Boudouresque et al., (Reference Boudouresque, Cadiou, Le Diréac'h and Levner2005).
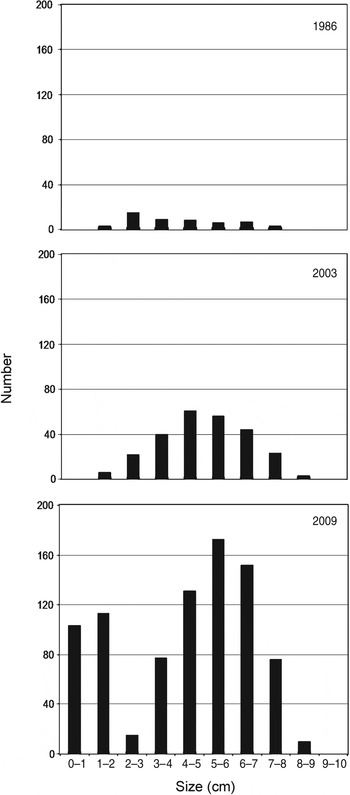
Fig. 5 Size (measured on the longitudinal axis) frequency distribution of P. ferruginea at Zembra in 1986 (adapted from Boudouresque & Laborel-Deguen, Reference Boudouresque, Laborel-Deguen, Boudouresque, Harmelin and Jeudy de Grissac1986), 2003 (adapted from Limam et al., Reference Limam, Rais and Ramos-Esplá2004) and 2009 (this study).
The distribution of P. ferruginea in the western Mediterranean is highly fragmented (Fig. 6). The status of the species is critical in continental France, where some individuals survive in the Port Cros marine protected area (Table 4; Cottalorda et al., Reference Cottalorda, Meinesz, Thibaut and Chiaverini2004), probably derived from Corsican populations via the marine currents that flow westward from Corsica to the Gulf of Lyon (Robinson et al., Reference Robinson, Leslie, Theocharis, Lascaratos, Turekian and Thorpe2001). The species is absent from the Mediterranean shores of the Iberian Peninsula (from the Gulf of Lyon to the Hormigas Islands; Espinosa et al., Reference Espinosa, Maestre and García-Gómez2009b), with the exception of some relict populations in Andalusia, mainly on the shores of Granada and Algeciras Bay (Espinosa et al., Reference Espinosa, Fa and Ocaña2005; Barba et al., Reference Barba, Nevado, Moreno, De La Linde, Remón and De La Rosa2006). The species was not considered rare in Algeciras Bay by García-Gómez (Reference García-Gómez1983), and Grandfils (Reference Grandfils1982) reported small populations along the promenades of Malaga and Fuengirola (Andalucía). However, recent data indicate that the species is scarce in the area and has disappeared from some sites. The estimated total number of individuals in the Iberian Peninsula is only c. 1,000 (Table 4). However, North African populations from the Strait of Gibraltar to Algeria exhibit high densities. The main populations are at Ceuta, Melilla, Chafarinas and the Habibas Islands (Table 4). Several sites have also been recorded in Mediterranean Morocco (from near Ceuta to close to the Chafarinas Islands): Midiq, Restinga, Cabo Negro, Oued Laou, El Jahba, Cabo Tres Forcas, Cabo del Agua and Essaidia (Bazairi & Benhissoune, Reference Bazairi and Benhissoune2004; Bazairi et al., Reference Bazairi, Salvati, Benhissoune, Tunesi, Rais and Agnesi2004; Espinosa, Reference Espinosa2006; Bazairi & Benhissoune, Reference Bazairi, Benhissoune and Paracuellos2007). Of these, the population in Al Hoceima National Park is important from a conservation perspective (Bazairi et al., Reference Bazairi, Salvati, Benhissoune, Tunesi, Rais and Agnesi2004). The populations in western Algeria (Rachgoun and Habibas islands) exhibit high densities, a well-structured size distribution and high reproductive output (Frenkiel, Reference Frenkiel1975; Boumaza & Semroud, Reference Boumaza and Semroud2001; Espinosa, Reference Espinosa2009). There is a lack of data from western Algeria to the Tunisian border, although isolated observations (very low density on Srigina island, Table 4; F. Bernard pers. comm.) indicate there are no significant populations in this area, consistent with the species' absence along the northern shores of Tunisia (Tlig-Zouari et al., Reference Tlig-Zouari, Rabaoui, Hosni and Ben Hassine2010; present study). Thus the range of the species in the western Mediterranean has contracted since the 19th century (Fig. 6). For example, Payraudeau (Reference Payraudeau1826) reported P. ferruginea as very common in Corsica but it is now rare.
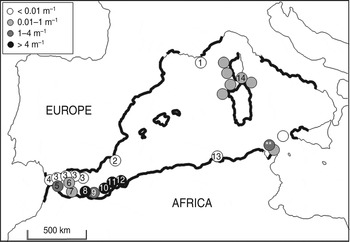
Fig. 6 Present locations (circles) of P. ferruginea based on recent reports and the results of our survey. Shading of circles represents density. Numbers in the circles correspond to the map numbers in Table 4; unnumbered circles indicate values obtained in this study (Table 1). Thicker lines along the coast indicate the range of the species in the 19th century (Laborel-Deguen & Laborel, Reference Laborel-Deguen, Laborel, Boudouresque, Avon and Gravez1991a).
Table 4 Summary of previous reports of the presence of P. ferruginea. Map numbers correspond to numbered locations in Fig. 6.

* Value from the most recent survey (Junta de Andalucía, Reference Junta De2010).
Although P. ferruginea is listed under European regulations and those of several Mediterranean countries it has not previously been assessed for the IUCN Red List. It could be categorized as Critically Endangered based on criteria A and E (IUCN, 2012). A population viability analysis (PVA) for the species indicated a decrease of >80% in the next 10 years (criterion A3) if the present collection rates continue at harvested sites (without larvae supply from well-preserved populations; Rivera-Ingraham et al., Reference Rivera-Ingraham, Espinosa and García-Gómez2011), and many populations of P. ferruginea have shown a decrease of >80% in the last 10 years (criterion A2), as reported by our study, including the populations of the southern Iberian Peninsula, Corsica, Italy, Egadi and Pantelleria islands and the Tunisian mainland. The PVA by Rivera-Ingraham et al. (Reference Rivera-Ingraham, Espinosa and García-Gómez2011) showed an extinction probability of >50% in the same period (criterion E). Their analysis was carried out on one of the most important relict populations (in Ceuta), and any PVA analysis of other small populations would probably reveal a greater extinction probability.
The fact that P. ferruginea occurs on artificial substrates throughout the western Mediterranean (Espinosa et al., Reference Espinosa, Rivera-Ingraham, Fa and García-Gómez2009a; García-Gómez et al., Reference García-Gómez, López-Fé, Espinosa, Guerra-García and Rivera-Ingraham2011) is critical when evaluating the potential threats to the species. When separated from demographic functionality, assessments of density may lead to incorrect conclusions about the value of artificial structures as alternative habitats because they may act only as ecological sinks in which organisms fail to breed successfully (Smallwood, Reference Smallwood2001). However, our study indicates that exploitation is the main factor influencing population structure and, consequently, potential population viability, and the type of substrate has no significant influence. In this context, a proposal for artificial marine micro reserves (García-Gómez et al., Reference García-Gómez, López-Fé, Espinosa, Guerra-García and Rivera-Ingraham2011) could be an effective conservation measure for P. ferruginea, protecting the relatively small areas of artificial substrates on which the species has settled. However, further studies are required to elucidate the population dynamics and ecological functioning of P. ferruginea populations on both artificial and natural substrates.
Acknowledgements
We are grateful to Fabrice Bernard, Sami Ben Haj and Sebastien Renou for logistical support under the initiative Petites Iles de Méditerranée of the Conservatoire du Littoral in the Zembra archipelago, to ‘Obimasa’ (Consejería de Medio Ambiente, Ciudad Autónoma de Ceuta) for their support, and to two anonymous referees for their useful comments. This study was partially funded by a predoctoral grant awarded to GAR-I by the Spanish Ministry of Education (FPU AP-2006-04220).
Biographical sketches
Free Espinosa is interested in the conservation of marine invertebrates and has been investigating Patella ferruginea for many years. Georgina Rivera-Ingraham has a long-standing interest in marine limpets and is now studying the response of marine species to hypoxia. Manuel Maestre is interested in the effects of human disturbance and substrate heterogeneity on marine biodiversity. Alexandre González focuses on monitoring and assessment of marine communities such as seagrass meadows and Mediterranean corals. Hocein Bazairi is interested in marine biodiversity and conservation and currently focuses on marine protected areas. Jose C. García-Gómez has worked on the biodiversity and conservation of marine ecosystems and is currently interested in long-term monitoring of coastal assemblages.












