Introduction
Data on the demography, ecological interactions and environmental requirements of Neotropical wildlife, and anthropogenic threats, have been used in the management of several species (e.g. Sanderson et al., Reference Sanderson, Redford, Chetkiewicz, Medellin, Rabinowitz, Robinson and Taber2002; Medici et al., Reference Medici, Desbiez, Gonçalves da Silva, Jerusalinsky, Chassot and Montenegro2007; ICMBio, 2015b). Although data accuracy and generality are paramount for conservation and management, most studies are context-dependent, concentrated either in regions dominated by pristine ecosystems or in medium to large protected areas (Fazey et al., Reference Fazey, Fischer and Lindenmayer2005). For threatened species, however, studies across landscapes modified and managed by humans are fundamental for the development of appropriate conservation policies (Chazdon et al., Reference Chazdon, Harvey, Komar, Griffith, Ferguson and Martínez-ramos2009).
Armadillos (Cingulata: Dasypodidae) are limited to the Neotropics (Wetzel, Reference Wetzel and Montgomery1985) and some species are sensitive to environmental change (Abba & Superina, Reference Abba and Superina2010). Of 11 species recorded in Brazil (ICMBio, 2015a), the giant armadillo Priodontes maximus is categorized as Vulnerable on the IUCN Red List (Anacleto et al., Reference Anacleto, Miranda, Medri, Cuellar, Abba and Superina2014). Populations of this armadillo are decreasing as a result of habitat loss, poaching, road-kills and the indiscriminate use of fire to remove natural vegetation or induce regrowth of pastures (Abba & Superina, Reference Abba and Superina2010; Martins et al., Reference Martins, Silva, de Fialho and Miranda2015). The lack of knowledge about the giant armadillo hinders the implementation of conservation actions, especially those focused on human-dominated landscapes (Meritt, Reference Meritt2006; Superina & Abba, Reference Superina and Abba2014). For example, although the predicted range of P. maximus extends from Venezuela to northern Argentina, including a large portion of Brazil (Anacleto et al., Reference Anacleto, Miranda, Medri, Cuellar, Abba and Superina2014; Chiarello et al., Reference Chiarello, Röhe, Miranda, Mourão, Silva, Vaz and Anacleto2015), it encompasses broad regions without records, and the species has been considered extinct in areas with high levels of urbanization/agricultural activities or without official records (Chiarello et al., Reference Chiarello, Aguiar, Cerqueira, Melo, Rodrigues, Silva, Machado, Drummond and Paglia2008; Srbek-Araujo et al., Reference Srbek-Araujo, Scoss, Hirsch and Chiarello2009). Despite its size (30–50 kg; Superina & Abba, Reference Superina and Abba2014) the giant armadillo is rarely observed because of its naturally low density and because it is nocturnal and fossorial (Noss et al., Reference Noss, Peña and Rumiz2004; Silveira et al., Reference Silveira, Jácomo, Furtado, Torres, Sollmann and Vynne2009; Srbek-Araujo et al., Reference Srbek-Araujo, Scoss, Hirsch and Chiarello2009; Aya-Cuero et al., Reference Aya-Cuero, Rodríguez-Bolaños and Superina2017). The absence of records of this species in highly modified areas could be a result of low survey effort rather than absence per se.
Central Brazil lies within the range of P. maximus (Anacleto et al., Reference Anacleto, Miranda, Medri, Cuellar, Abba and Superina2014; Chiarello et al., Reference Chiarello, Röhe, Miranda, Mourão, Silva, Vaz and Anacleto2015) and is dominated by the Cerrado biome, with the Atlantic Forest biome represented by enclaves of seasonal forest along watercourses (e.g. the Paranaíba river basin; Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009). As a result, there is a rich mosaic of physiognomies, ranging from open grasslands to forest patches, favouring a high regional biodiversity (Lopes et al., Reference Lopes, Schiavini, Oliveira and Vale2012). However, agricultural activities and urbanization have resulted in the replacement of > 50% of the natural vegetation by anthropogenic environments (Machado et al., Reference Machado, Ramos-Neto, Pereira, Caldas, Gonçalves and Santos2004). The landscape now comprises a matrix of exotic pastures and crops surrounding fragments of natural vegetation, the latter located mainly on higher slopes or in rugged terrain (Klink & Machado, Reference Klink and Machado2005; Carvalho et al., Reference Carvalho, Marco-Júnior and Ferreira2009). The occurrence of P. maximus in central Brazil remains poorly known (Martins et al., Reference Martins, Silva, de Fialho and Miranda2015). Here, we present records of the giant armadillo in modified landscapes of the Cerrado biome and ecotone areas with the Atlantic Forest biome in the border area of the states of Goiás and Minas Gerais. We also present data on the species’ activity period and its habitat use in agroecosystems.
Study area
Our study area lies in 10 municipalities of Goiás and Minas Gerais States (Supplementary Table 1; Fig. 1), in a region where 70–80% of the area is occupied by cattle ranches with exotic pastures, and the remainder comprises scattered natural patches of savannah (Cerrado) and mesophytic seasonal forest (Atlantic Forest). The regional climate is markedly seasonal (Alvares et al., Reference Alvares, Stape, Sentelhas, Gonçalves and Sparovek2013), with a mean annual temperature of 23–25 °C and mean annual total precipitation of 1,600–1,900 mm.

Fig. 1. Records of the giant armadillo Priodontes maximus in 10 municipalities in the states of Goiás and Minas Gerais, Brazil. Municipalities: 1, Água Limpa; 2, Caldas Novas; 3, Ipameri; 4, Urutaí; 5, Campo Alegre; 6, Catalão; 7, Goiandira; 8, Cumari; 9, Araguari; 10, Uberaba; 11, Perdizes. Previous records are from Martinelli et al. (Reference Martinelli, Costa, Neto, Fonseca, Martins and Silva2014), Araújo et al. (Reference Araújo, Silva, Estrela and Castro2015), Chiarello et al. (Reference Chiarello, Röhe, Miranda, Mourão, Silva, Vaz and Anacleto2015), Estrela et al. (Reference Estrela, Souza, Souza and Castro2015), Gomes et al. (Reference Gomes, Rocha, Brandão and Marinho-Filho2015), and Rocha et al. (Reference Rocha, Soares and Pereira2015).
Methods
Data on P. maximus occurrence were obtained during 2003–2016 in natural vegetation remnants in farmlands, private reserves, and protected areas, from camera trapping (Plate 1a), sightings (Plate 1b), tracks (Plate 2a) and fresh burrows (Plate 2b). We did not include old, abandoned burrows or those where there was evidence they had been used by other species (Desbiez & Kluyber Reference Desbiez and Kluyber2013). Camera-trap surveys were in private natural vegetation remnants in the municipalities of Araguari (Minas Gerais; 2009–2016), in pastures with dense bushes in Cumari (Goiás; 2003–2016), in the Serra de Caldas Novas State Park (Caldas Novas, Goiás; 2010–2011), and in the private reserve Pé do Morro Farm of the Universidade Federal de Goiás (Catalão, Goiás; 2014–2015). Camera-trap records in different months at each location were considered separately. To determine if camera-trap photographs were taken during the day or night we calculated the time of sunrise and sunset for the location and date of each photograph, using R v. 2.6 (R Development Core Team, 2014). We also included data from road-kills (Plate 3a) and poaching by local people, reported voluntarily during occasional visits to farms (Plate 3b). We compared our findings with those of previous studies (Martinelli et al., Reference Martinelli, Costa, Neto, Fonseca, Martins and Silva2014; Araújo et al., Reference Araújo, Silva, Estrela and Castro2015; Chiarello et al., Reference Chiarello, Röhe, Miranda, Mourão, Silva, Vaz and Anacleto2015; Estrela et al., Reference Estrela, Souza, Souza and Castro2015; Gomes et al., Reference Gomes, Rocha, Brandão and Marinho-Filho2015; Rocha et al., Reference Rocha, Soares and Pereira2015; Fig. 1).

Plate 1. Giant armadillos Priodontes maximus recorded by (a) camera trapping and (b) sighting in Pé do Morro Farm reserve and Serra de Caldas Novas State Park (respectively), in the state of Goiás (Fig. 1).

Plate 2. Typical evidence of the giant armadillo used to record the species: (a) track and (b) fresh burrow on a leaf-cutter ant nest.
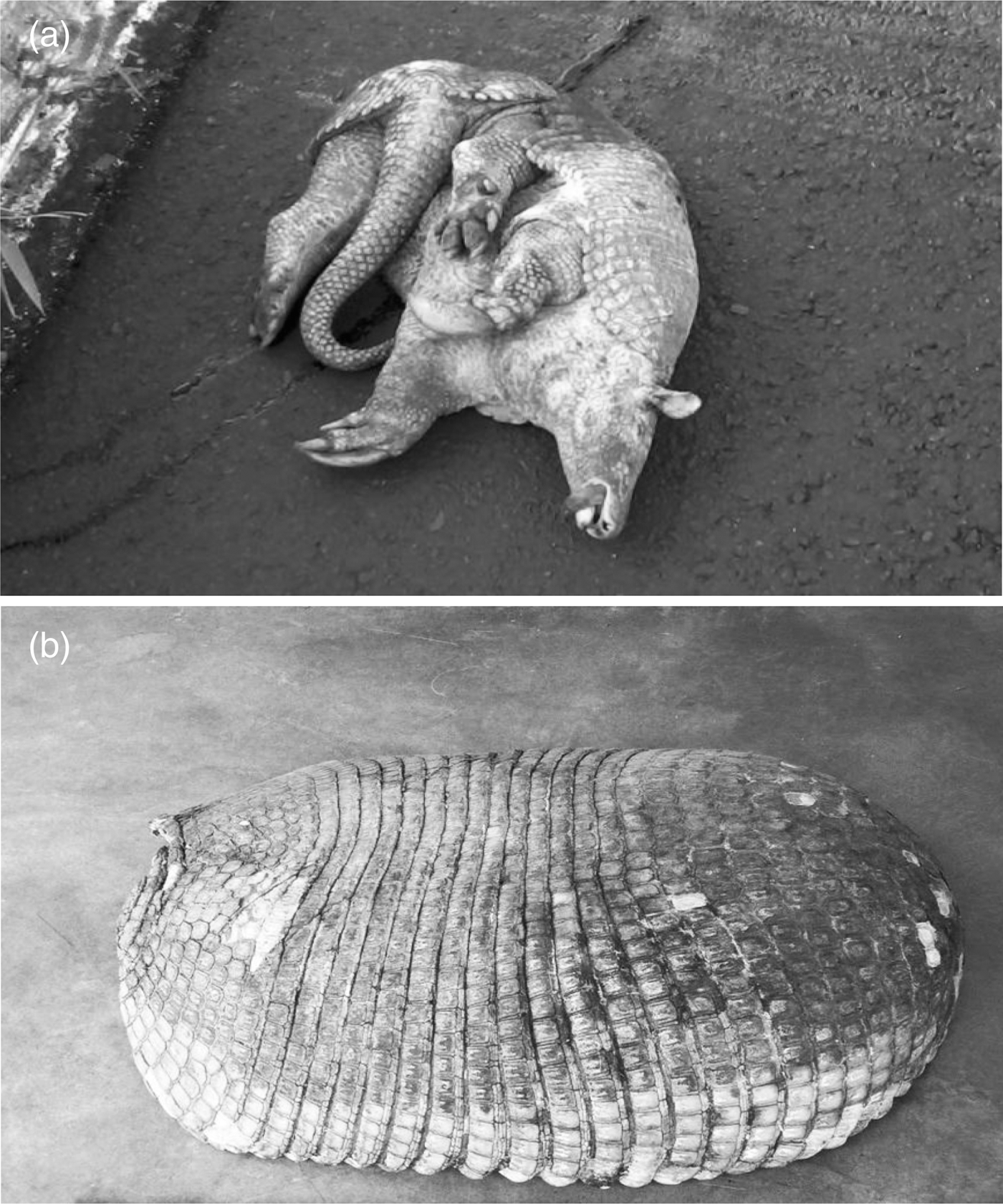
Plate 3. Carcasses of the giant armadillo recorded in the state of Goiás: (a) killed on the GO-330 highway, and (b) the carapace of a poached animal.
To determine habitat use we classified the vegetation in each location as savannah, forest or pasture. Savannah has a relative predominance of native grasses and scattered shrubs and small trees, which in the Cerrado includes the vegetation physiognomies campo sujo, campo cerrado and cerrado sensu stricto. Forest consisted of trees > 12 m tall and with canopy formation such as the cerradão physiognomy, seasonal forests, riverine forests or gallery forests (Oliveira-Filho & Ratter, Reference Oliveira-Filho, Ratter, Oliveira and Marquis2002). To assess differences in proportions of used and available habitat we compared the frequency of records and available habitat for the agro-ecosystems surveyed in Araguari (Minas Gerais; F.C. Azevedo unpubl. data) and Cumari (Goiás; F.G. Lemos unpubl. data), and the reserve Pé do Morro Farn in Catalão (Goiás; Cardoso & Moreno, Reference Cardoso and Moreno2013).
Results
During the 14-year period we obtained a total of 54 records of P. maximus: 27 from 16 private farmlands, 12 in a private reserve, 10 in three protected areas, three animals killed on paved roads and two poached (Fig. 1; Supplementary Table 1). Forty-six per cent of the records were from camera trapping (17), carcasses (5) and sightings (3), and 54% (29) were from fresh burrows and tracks, often on trails or at the edge of dirt roads.
Camera-trap photographs and sightings were almost exclusively during the night (Fig. 2), with just one in daylight, shortly after dawn (Supplementary Table 1). Most records were in areas with native vegetation cover (83%), with 24 in forest and 21 in savannah. In Cumari and Catalão six records were in pastures. The frequency of records in different habitats generally followed their relative availability in each study site (Table 1). The 17% of records in anthropogenic environments, including road-kills, were < 0.45 km from natural vegetation remnants.

Fig. 2. Activity pattern of the giant armadillo, based on 20 camera-trap photographs and three sightings during July 2010–October 2016 in four municipalities in the states of Goiás and Minas Gerais (Fig. 1).
Table 1 Availability of habitats and number of records (from camera trapping, sightings, carcass recovery, tracks and burrows) of the giant armadillo Priodontes maximus during 2009–2016 in the municipalities of Araguari (Minas Gerais) and Cumari (Goiás), and the reserve Pé do Morro Farm in Catalão (Goiás).
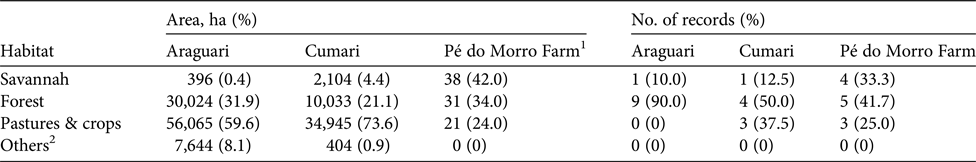
1 Data from Cardoso & Moreno (Reference Cardoso and Moreno2013).
2 Urban areas, farm facilities, roads, artificial dams and degraded areas.
Discussion
The giant armadillo is widely distributed in central Brazil (Anacleto et al., Reference Anacleto, Miranda, Medri, Cuellar, Abba and Superina2014; Chiarello et al., Reference Chiarello, Röhe, Miranda, Mourão, Silva, Vaz and Anacleto2015), and elderly rural residents reported to us that formerly it was not unusual to find this armadillo's tracks or to sight the species on their properties. However, the giant armadillo is now rarely seen, most likely as a result of habitat loss combined with road-kills and poaching (Chiarello et al., Reference Chiarello, Aguiar, Cerqueira, Melo, Rodrigues, Silva, Machado, Drummond and Paglia2008). Intensive mammal surveys have only recently been conducted in natural vegetation remnants across the study region (Bruna et al., Reference Bruna, Guimarães, Lopes, Duarte, Gomes and Belentani2010; Araújo et al., Reference Araújo, Silva, Estrela and Castro2015; Estrela et al., Reference Estrela, Souza, Souza and Castro2015; Gomes et al., Reference Gomes, Rocha, Brandão and Marinho-Filho2015; Rocha et al., Reference Rocha, Soares and Pereira2015). Our study spans the longest period (14 years) and covered 192 km2 of Cerrado and Atlantic Forest remnants.
Protected areas on the border of Minas Gerais and Espirito Santo states are considered the last stronghold for P. maximus in the Atlantic Forest (Srbek-Araujo et al., Reference Srbek-Araujo, Scoss, Hirsch and Chiarello2009) but our findings indicate that the species is still present in Atlantic Forest remnants within the basins of the Paranaíba and Araguari rivers. In addition, a record from Vale do Encantado Private Natural Heritage Reserve in the municipality of Uberaba (Minas Gerais) expands the southern range of the species (Martinelli et al., Reference Martinelli, Costa, Neto, Fonseca, Martins and Silva2014; Fig. 1). Previous studies in south-east Goiás state located only six records of P. maximus for 2004–2014 (Araújo et al., Reference Araújo, Silva, Estrela and Castro2015; Chiarello et al., Reference Chiarello, Röhe, Miranda, Mourão, Silva, Vaz and Anacleto2015; Estrela et al., Reference Estrela, Souza, Souza and Castro2015; Gomes et al., Reference Gomes, Rocha, Brandão and Marinho-Filho2015), but we found 42 records within the same general area. Based on the home range size of a giant armadillo (500–1,500 ha; Silveira et al., Reference Silveira, Jácomo, Furtado, Torres, Sollmann and Vynne2009) we assume that at least one animal was living in the vegetation remnants surrounding the record points, and thus our findings, in combination with previous records, potentially indicate the persistence of several populations of this species in natural patches across a large section of agro-ecosystems in central Brazil. However, there are many potentially suitable vegetation remnants within the area that remain unsurveyed.
Our findings corroborate previously identified traits of giant armadillos: individuals are essentially nocturnal (Noss et al., Reference Noss, Peña and Rumiz2004; Silveira et al., Reference Silveira, Jácomo, Furtado, Torres, Sollmann and Vynne2009; Srbek-Araujo et al., Reference Srbek-Araujo, Scoss, Hirsch and Chiarello2009; Aya-Cuero et al., Reference Aya-Cuero, Rodríguez-Bolaños and Superina2017), occur in both open savannah and closed forest habitats, and their use of habitat tends to reflect habitat availability in the landscape (Silveira et al., Reference Silveira, Jácomo, Furtado, Torres, Sollmann and Vynne2009). It is most likely that giant armadillos select areas with populations of termites or ants, which are their main food items (Anacleto, Reference Anacleto2007), independently of the dominant vegetation of an area.
Although we surveyed extensive areas of pasture in the matrix between natural vegetation patches, the low frequency of records (11%) in this environment suggests that exploration of human-modified habitats is uncommon for giant armadillos even in predominantly altered landscapes; they mainly explore natural vegetation patches within these highly modified areas (Silveira et al., Reference Silveira, Jácomo, Furtado, Torres, Sollmann and Vynne2009). Similarly, the bush dog Speothos venaticus tends to use native vegetation preferentially (95% of records in savannah and forest patches), even where this vegetation occupied only one-third of a cultivated landscape in Mato Grosso state (Lima et al., Reference Lima, Jorge, Jorge and Morato2015). This highlights the importance of the protection of private reserves and biological corridors, mainly gallery forests, for the conservation of rare and threatened species in modified landscapes.
Habitat fragmentation often results in vegetation patches decreasing in size and increasing in number and isolation (Fahrig, Reference Fahrig2003), negatively affecting species with large spatial requirements such as the giant armadillo (Chiarello, Reference Chiarello1999). However, 28 of our records were from fragments smaller (25–288 ha) than an armadillo's home range and therefore potentially unsuitable to support even a single giant armadillo. This could be explained by an extinction debt (Kuussaari et al., Reference Kuussaari, Bommarco, Heikkinen, Helm, Krauss and Lindborg2009) for the giant armadillo. The species’ relatively long life expectancy (12–15 years; Nowak, Reference Nowak1999) indicates low population turnover and may mask the long-term effects of fragmentation. The irregular topography of the landscape in the border areas between Goiás and Minas Gerais states results in habitat remnants being close to each other, allowing giant armadillos to forage in small areas and still survive within fragmented landscapes for a limited time. This extinction delay could present an opportunity for recovery of this species via habitat restoration and landscape management (Kuussaari et al., Reference Kuussaari, Bommarco, Heikkinen, Helm, Krauss and Lindborg2009).
Road-kills of armadillos and other mammal species have been recorded in central Brazil (Fischer et al., Reference Fischer, Ramos-Neto, Silveira, Jácomo, Irwin, Garret and McDermott2003), and we located three road-kills, but this problem is probably under-reported. Illegal hunting is still a common practice in central Brazil and game meat consumption remains a cultural habit, mainly in rural communities (FGL pers. comm.), although probably insignificant compared to that observed in the Amazon forest (e.g. Baía-Junior et al., Reference Baía-Junior, Guimarães and Le Pendu2010; van Vliet et al., Reference van Vliet, Quiceno-Mesa, Cruz-Antia, de Aquino, Moreno and Nasi2014). The low frequency of hunting in agricultural regions (Rushton et al., Reference Rushton, Viscarra, Viscarra, Basset, Baptista and Brown2005) such as central Brazil may be associated with access to domestic sources of animal protein and stricter environmental laws. We recorded only two poached giant armadillos, and suspect that hunting of this species in central Brazil is opportunistic.
The decline of the giant armadillo (at least 30% since 1991; ICMBio 2015a) may influence community diversity and vegetation structure. The species is an ecosystem engineer (Leite-Pitman et al., Reference Leite-Pitman, Powell, Cruz, Escobedo, Escobar, Vilca and Mendoza2004; Desbiez & Kluyber, Reference Desbiez and Kluyber2013; Aya-Cuero et al., Reference Aya-Cuero, Rodríguez-Bolaños and Superina2017), a potential prey for large predators such as the jaguar Panthera onca and cougar Puma concolor, and a specialized insect-predator (Anacleto, Reference Anacleto2007). With respect to the latter, the absence of a top-down effect on insect herbivores, especially leaf-cutter ants (Costa & Vieira-Neto, Reference Costa and Vieira-Neto2016), may affect vegetation structure and dynamics in modified environments (Terborgh et al., Reference Terborgh, Lopez, Nuñez, Rao, Shahabuddin and Orihuela2001; Silva et al., Reference Silva, Leal, Wirth, Melo and Tabarelli2012). The border region of the states of Goiás and West Minas Gerais has experienced intensive landscape modification, and < 2–3% of natural vegetation remnants lie within protected areas (Carvalho et al., Reference Carvalho, Marco-Júnior and Ferreira2009). The high degree of landscape modification and the high cost of land in this region are challenges to the establishment of protected areas large enough to ensure the conservation of large mammals. Changes in 2011 and 2012 to the Brazilian Forest Act, providing amnesty for illegal logging, allowing mandatory legal reserve areas to include sites previously prohibited from being deforested, and reducing the deforestation-free zone beside rivers, contribute to the decrease of biological connections between natural vegetation remnants and potentially result in biodiversity loss (Michalski et al., Reference Michalski, Norris and Peres2010; Paul et al., Reference Paul, Lewinsohn, Joly, Luciano, Martinelli and Rodrigues2010).
Our research suggests that the giant armadillo may still occur in other highly modified areas of central Brazil and elsewhere, undetected because of low survey efforts. This may also be the case for other rare and threatened Brazilian species (e.g. bush dogs were recently recorded in our study area, where the species was expected but had not previously been observed; Azevedo et al., Reference Azevedo, Lemos, Rocha, Costa and de Freitas-Junior2016). Our results suggest that road-kills and poaching, in addition to habitat loss, continue to threaten the giant armadillo in central Brazil. Policies that both deter illegal deforestation and strengthen incentives for the protection of natural vegetation remnants and restoration of biological corridors such as gallery forests are therefore necessary for the conservation of the giant armadillo in this area.
Acknowledgements
We thank Hugo Costa, Frederico Cigano Souza, Leandro Abade, Guilherme Dias, Lucas Ribeiro and Tiago Borges for their assistance with fieldwork, Daniel Rocha for helping with time calculations, Leonardo Gomes for providing coordinates for mapping literature records, Stacie Castelda-Bickley, Arnaud Desbiez, Ernane Vieira-Neto, Martin Fisher and anonymous reviewers for their critiques, landowners and protected area managers for their hospitality, Capim Branco Energy Company for financial support for part of the study and Minas Gerais Electrical Company for the permit to work at Galheiro Private Natural Heritage Reserve. FCA was supported by a doctoral fellowship from HIDROEX (UNESCO/BID), and ECR by a grant from Goiás State University (PROBIP/PrP 009/2016).
Author contributions
Conception and design of the study: FGL, ANC, FCA, CEF and ECR; data collection: FGL, ANC, FCA, CEF, MCF and ECR; writing: FGL, ANC, FCA and CEF; revisions: FGL, ANC, FCA, CEF, MCF and ECR.
Conflicts of interest
None.
Ethical standards
This study abided by the Oryx Code of Conduct.








