Introduction
The Hawaiian archipelago was formerly home to one of the most species-rich land snail faunas (> 752 species; Cowie et al., Reference Cowie, Evenhuis and Christensen1995; Yeung & Hayes, Reference Yeung and Hayes2018). This rich fauna resulted primarily from in situ speciation, leading to levels of endemism > 99% (Cowie, Reference Cowie1995). Unfortunately, many Hawaiian land snail species are extinct (Yeung & Hayes, Reference Yeung and Hayes2018), and the remaining fauna, particularly ground-dwelling species, have been severely impacted. For example, only three of the estimated 300 endodontid species and 21 of 325 amastrid species remain (Yeung & Hayes, Reference Yeung and Hayes2018; Yeung et al., Reference Yeung, Bustamente, Sischo and Hayes2018; Hayes et al., Reference Hayes, Slapcinsky, Sischo, Kim and Yeung2020). Although only nine of the 42 species in the genus Achatinella, the well-publicized Oʻahu tree snails listed as endangered under the Endangered Species Act (USFWS, 1981), remain, other native Hawaiian arboreal snails have fared better, particularly smaller (< 5 mm) achatinellids, succineids, and euconulids (Yeung & Hayes, Reference Yeung and Hayes2018). However, reductions in range sizes and population densities of all extant species highlight the urgent need for effective conservation strategies (Solem, Reference Solem1990; Yeung & Hayes, Reference Yeung and Hayes2018).
Information on how resources influence the abundance and distribution of invertebrate species is often limited (Cardoso et al., Reference Cardoso, Erwin, Borges and New2011). For most Hawaiian snails, ecological information on critical habitat requirements is unknown (Solem, Reference Solem1990). Understanding which plant species are preferred hosts for arboreal snails is key to determining which combination of native plant species can facilitate preservation of native snail diversity, and alternatively, how changes in abundance of native plant species can influence native snail populations (Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014). Preferred host plants are determined by identifying which plants had more snails on them than expected by chance. Understanding the snails' preferences is the foundation required for further studies to test the mechanisms that underlie these preferences.
The purposes of this study were to: (1) examine the plant preferences of the native arboreal land snails in the Puʻu Kukui Watershed Preserve (hereafter, Puʻu Kukui; owned by Maui Land & Pineapple Company Inc.) on Mauna Kahalawai (West Maui), Hawaiʻi (Fig. 1), and (2) compare these results to similar studies on Oʻahu (Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014) and the Island of Hawaiʻi (Meyer, Reference Meyer2012) to examine if concordant patterns exist across islands. Puʻu Kukui is home to at least 40 extant native land snail species, most being arboreal (K.A. Hayes et al., unpubl. data), and the flora at higher elevations comprises primarily native species (Table 1). Studies in native forests are valuable for informing land snail conservation because these forests are where the remaining native snail species are found (Meyer & Cowie, Reference Meyer and Cowie2010), and provide an ecological context for understanding interactions among native snails and plants (Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014).
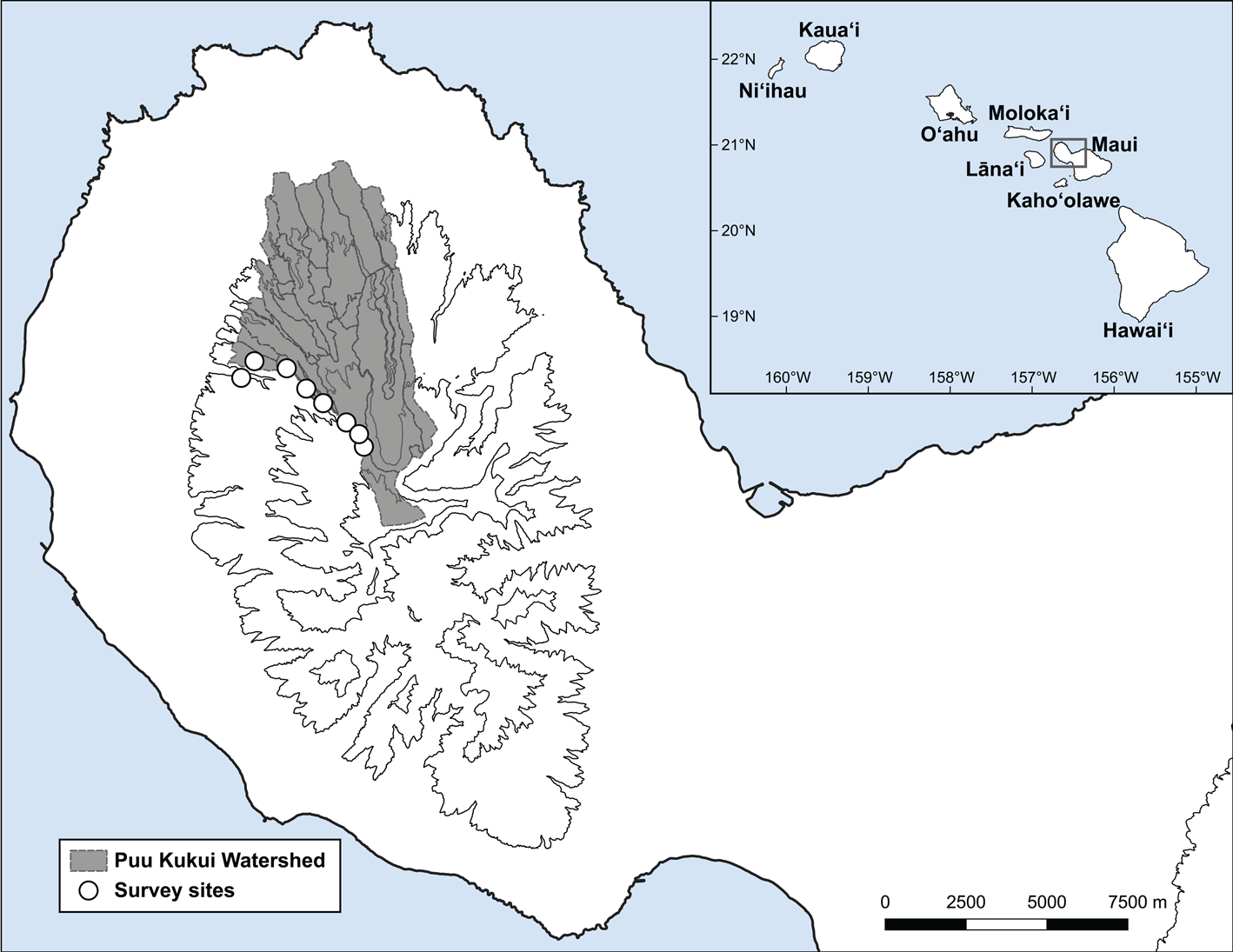
Fig. 1 Puʻu Kukui Watershed Preserve (shaded region) located on Maui Island, showing the nine survey sites (white circles) along an elevation gradient of 730–1,380 m. The inset map shows the main Hawaiian Islands.
Table 1 Plant selection by snails in Puʻu Kukui Watershed, Maui, in descending order by mean per cent cover. Values > 0 indicate preference, and values < 0 indicate avoidance. Preference values > 0.25 are in bold.
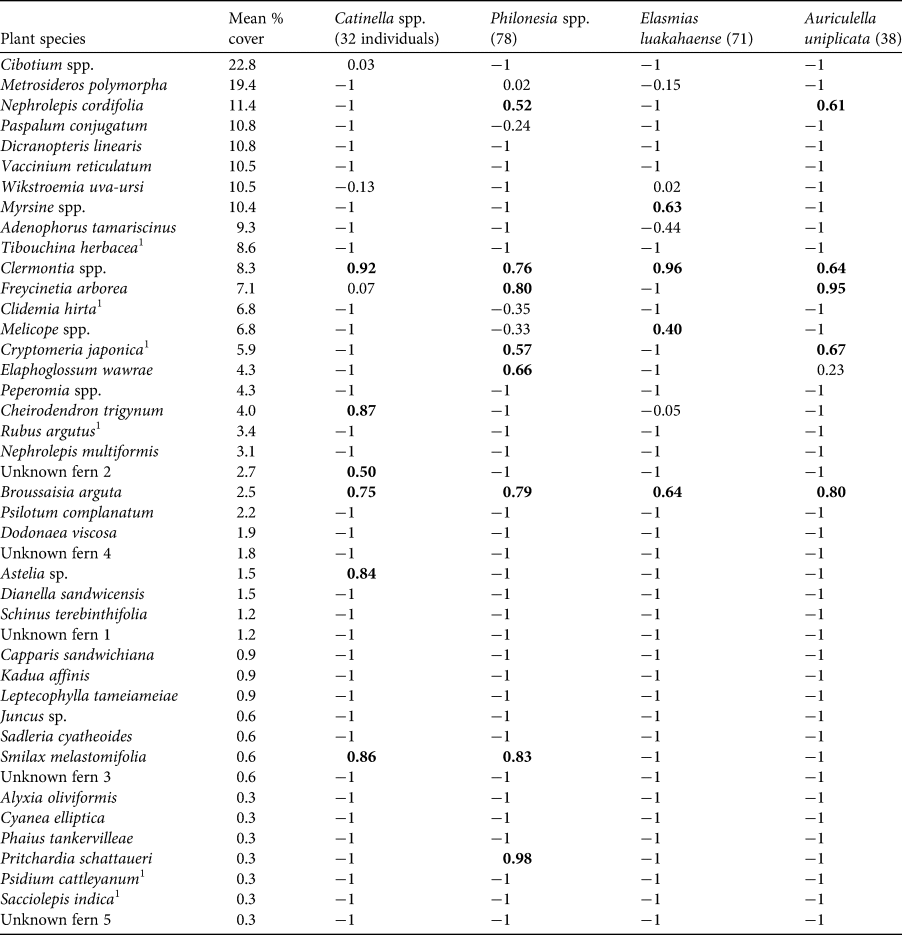
1 Non-native species.
Studies examining plant preferences of arboreal snails on Oʻahu and Hawaiʻi found that a diversity of native snails demonstrated strong preferences for a small proportion of plant species present (Meyer, Reference Meyer2012; Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014). Explanations for such selectivity have largely focused on differing food (epiphytic fungi and bacteria: the phyllosphere) quality among plant species, and/or differing plant traits, such as robust (thick and/or static in varying conditions to provide protection from the rain) and smooth leaves that allow access to the phyllosphere and attachment of the aperture and epiphragm during estivation (Brown et al., Reference Brown, Spain and Crowell2003, Reference Brown, Spain and Arizumi2006; Meyer, Reference Meyer2012; Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014; O'Rorke et al., Reference O'Rorke, Cobian, Holland, Price, Costello and Amend2015; Holland et al., Reference Holland, Chiaverano and Howard2017; Sato et al., Reference Sato, Price and Vaughan2018). Using previous studies that examined plant preferences (Brown et al., Reference Brown, Spain and Crowell2003, Reference Brown, Spain and Arizumi2006; Meyer, Reference Meyer2012; Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014), we hypothesized that arboreal snails in Puʻu Kukui prefer a small subset of native plant species with robust and smooth leaves, and we predicted that Broussaisia arguta, a large-leafed native shrub, would be preferred by native snails in Puʻu Kukui.
Methods
To test these hypotheses, nine 5 × 5 m quadrats were surveyed in Puʻu Kukui over 2 days (14–15 June 2018; Fig. 1) by nine experienced malacologists (all authors except JRK). Quadrats were distributed along an elevation gradient of 730–1,380 m, at least 100 m apart. To determine the per cent cover of each plant species, we used the point intercept method, which is more precise and repeatable than visual cover estimates (Godinez-Alvarez et al., Reference Godinez-Alvarez, Herrick, Mattocks, Toledo and van Zee2009). Each quadrat contained four parallel 5 m transects separated by 1 m, with the first 1 m from the edge. To estimate per cent cover for each plant species, a ¾ inch (c. 1.9 cm) diameter pole was positioned perpendicular to the ground at every 0.5 m along each transect. We recorded every plant species that the pole touched at each point for a total of 36 points per quadrat (we did not sample points at the edge of the transect; i.e. at 0 or 5 m).
All additional plant species not recorded in the point intercept surveys, but present in the quadrat, were then recorded. We assigned all plants to morphospecies and identified all plants to the lowest taxonomic level possible, in most cases to species. Following plant surveys, we surveyed all plants for snails (top and bottom of leaves, stems and trunks). Most plants were < 2 m in height, allowing researchers to survey the whole plant. Plants > 2 m height were surveyed only up to 2 m. At the start of each survey, all surveyors were assigned a plant species, and collected all the snails from this species in the quadrat, recording the number of individuals of each snail taxon on that plant before being assigned another plant species. This was repeated until all plant species had been surveyed. Following surveys of all plants in a quadrat, individual snails were identified to the lowest taxonomic level possible, in most cases to species. Representatives of native snail morphospecies were collected, preserved, and deposited in the Bishop Museum, Honolulu (Supplementary Table 1). Native snails not collected for further taxonomic study were released back into the plots. Non-natives were either collected or euthanized.
To estimate the plant preferences of each snail species, we used the Jacobs' selectivity index (Jacobs, Reference Jacobs1974), calculated as:
where Dia is the selectivity index of snail species i for plant type a, r i is the ratio of plant type a use to all other plant types used, and p a is the ratio of plant a to all other plants. The index ranges from −1 to 1. Values > 0 indicate preference (proportion of snails on that species was higher than the relative per cent cover of the plant species). Values below zero indicate avoidance. Because both plant and snail species were patchily distributed, we calculated a global Jacobs' selectivity index for each snail species using mean per cent cover of all plant species and the proportional number of individuals of each snail species on each plant species across all plots (Table 1). To be conservative, we examined preference for snail species only if > 30 individuals were recorded, and we identified preference when Jacobs’ index was > 0.25.
Results
We recorded 283 individual snails, but identifying most specimens to species in the field was not possible, and therefore individuals were assigned to 10 taxonomic groupings that were readily identifiable during surveys. The seven native taxonomic groups were Catinella spp., Philonesia spp., Auriculella uniplicata, Elasmias luakahaense, Punctum sp., Lamellidea cf. polygnampta, and Tornatellidinae spp. (Supplementary Table 2). Non-native groupings were Oxychilus alliarius, Deroceras laeve and Deroceras reticulatum. Four taxonomic groupings (hereafter, species: Catinella spp., Philonesia spp., A. uniplicata and E. luakahaense) were sufficiently abundant for preference analyses.
Consistent with previous studies on Oʻahu and the island of Hawaiʻi, evolutionarily distant snails had relatively similar plant preferences (Table 1). For example, two plant species, B. arguta and Clermontia spp. were preferred by all four snail species. Consistent with our hypothesis, all preferred plant species are understorey plants. The dominant native tree species, Metrosideros polymorpha, which formed the canopy in many of the sites surveyed, and the common native mid canopy shrub, Cibotium spp., were not preferred.
Discussion
Concordant patterns in arboreal snail preferences across islands provide valuable insights into management strategies that may benefit snail conservation in the Hawaiian Islands. Firstly, arboreal snail preferences for a few species of understorey plants, and not the most widespread and abundant canopy and mid canopy species (M. polymorpha and Cibotium spp.) indicate that native forests without key understorey plant species may not support native snails. Secondly, consistent preference for B. arguta among various island endemic snails on three islands (Brown et al., Reference Brown, Spain and Crowell2003, Reference Brown, Spain and Arizumi2006; Meyer, Reference Meyer2012; Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014) indicates that this plant species is critically important in restoration to improve snail habitat. Other important native understorey species can also be identified by comparing studies. For instance, Smilax melastomifolia was a preferred plant species for two of the four snail species in our study, and four of the six snail species studied on Oʻahu (Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014). However, patterns within just one site may also be important, particularly if the plant species was preferred by many snail species. Ilex anomala was a preferred species of all snails examined on Oʻahu, but no Ilex species were recorded in our study. Similarly, preference for Clermontia spp. had not been previously reported, but Clermontia spp. are sensitive to browsing mammals, and as such are rare, patchily distributed, and thus not likely to be recorded in previous experimental plots (Medeiros et al., Reference Medeiros, Loope and Holt1986). Anecdotally, many native succineids were consistently found on two Clermontia individuals near Meyer's (Reference Meyer2012) transects on the island of Hawaiʻi. As such, restoration efforts to enhance populations of these threatened plant species, and other understorey plants, may also benefit snails, and potentially other native invertebrates.
Understanding the plant preferences of snails not only provides insight into which combination of native plant species may facilitate preservation of native snail diversity, but also highlights that losses or changes in abundance of native plant species can influence extant native snail populations. There has been no evaluation of how rapid reductions in the ranges of many Hawaiian plant species over the last century (Burney & Burney, Reference Burney and Burney2007) have affected native snail species. Even in areas not threatened by human disturbance, rooting and trampling by non-native pigs, and other non-native ungulates, significantly reduces native understorey plant richness and cover (Cole & Litton, Reference Cole and Litton2014). In addition to reducing understorey plants, non-native plant species are often the first to colonize areas disturbed by pigs (Aplet et al., Reference Aplet, Anderson and Stone1991), further changing the understorey plant community. Non-native plants are not often used by native snails, although invasive ginger species (Hedychium spp.) on the island of Hawaiʻi are a preferred plant of native succineid snails (Brown et al., Reference Brown, Spain and Crowell2003, Reference Brown, Spain and Arizumi2006; Meyer, Reference Meyer2012). Identifying plants key to snail survival is a critical step in stemming the loss of this highly threatened fauna and provides important insights to help identify areas to protect and to restore areas to provide habitat for the remaining arboreal snails. This is fundamental information for areas where arboreal snails are present, reintroductions are being considered, and in enclosures built to protect native snails from introduced predators. Although additional research is required to quantify how snail fitness is influenced by various native plant species, consideration of the composition of plant assemblages could be a key management strategy for providing habitat to maintain stable arboreal snail populations.
Concordant patterns across islands help to indicate which plant species are critical for snail restoration but it is difficult to ascertain if leaf traits are good predictors of snail preferences. Although smooth ferns, Elaphoglossum wawrae and Dryopteris sp., were preferred, and the hairy Cibotium spp. were avoided on Maui, as on Oʻahu and Hawaiʻi (Meyer, Reference Meyer2012; Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014), consistent patterns across all plants are difficult to identify. For example, some common plants with both robust and smooth leaves, most notably, M. polymorpha, are not preferred at Puʻu Kukui or on other islands (Meyer, Reference Meyer2012; Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014), but may be a preferred species for Achatinella spp. (O'Rorke et al., Reference O'Rorke, Cobian, Holland, Price, Costello and Amend2015; Holland et al., Reference Holland, Chiaverano and Howard2017; Sato et al., Reference Sato, Price and Vaughan2018). Similarly, although many plant species preferred by snails could be considered smooth (e.g., Clermontia spp., Freycinetia arborea, Astelia sp., S. melastomifolia), high numbers of Philonesia spp. on Cryptomeria japonica, a non-native cedar, were unexpected, as most were found on the rough bark. In addition, determining if Pritchardia schattaueri, a native Hawaiian palm, should be considered robust, probably depends on ecological context. Fronds of P. schattaueri can be heard crashing into one another in times of elevated winds, but snails are typically found on palms in protected valleys where winds are low. An objective examination of leaf trait preferences requires that all plant traits be defined before a survey is conducted, and analyses test if snails were found on plants with certain traits more than expected by chance. As this has not been done, our current understanding of which leaf traits are important are only anecdotal. We recommend field assessments be used to determine which plants snails prefer prior to any conservation action.
Synthesizing results from studies that examined snail feeding and host plant preferences highlights that our knowledge of what constitutes critical habitat for most snail species is still limited. For example, although O'Rorke et al. (Reference O'Rorke, Cobian, Holland, Price, Costello and Amend2015) found that the phyllosphere did not differ among plant species within a site, they did not assess the phyllosphere of plants that did not serve as snail hosts. As such, it remains unclear whether snails choose host plants based on phyllosphere assemblages. Holland et al. (Reference Holland, Chiaverano and Howard2017) found that one native snail species had reduced fitness when provided non-native plants compared to native plants. Plant species used by Holland et al. (Reference Holland, Chiaverano and Howard2017) were not examined by O'Rorke et al. (Reference O'Rorke, Cobian, Holland, Price, Costello and Amend2015), limiting comparisons between the two studies. Also, the findings of Holland et al. (Reference Holland, Chiaverano and Howard2017) contrast with studies examining plant preferences in the Hawaiian Islands. For example, a non-native ginger species, Hedychium coronarium, used in the feeding trials, was preferred by native succineids on Hawaiʻi and reproduction on ginger in the wild seems robust (Brown et al., Reference Brown, Spain and Crowell2003, Reference Brown, Spain and Arizumi2006; Meyer, Reference Meyer2012). However, Hedychium spp. were not utilized by snails on Oʻahu (Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014). Hedychium spp. were rare on Oʻahu, making preference assessment for these species difficult. Also, M. polymorpha, a widespread native tree species used as one of the native species by Holland et al. (Reference Holland, Chiaverano and Howard2017), was not preferred by all snail species in wet forests on Maui (Puʻu Kukui), Hawaiʻi or Oʻahu (Meyer, Reference Meyer2012; Meyer et al., Reference Meyer, Gary, Yeung, Dirks, Leung and Léon2014). An approach that uses the same plant species and snail taxonomic groupings and incorporates snail plant preference observations, examination of phyllosphere differences among plant species, and laboratory rearing studies is required to elucidate whether differences in food resources underlie plant preferences and influence snail fitness.
Only through studies of the ecology of Hawaiian land snails can informed decisions be made regarding which management methods could be used to conserve the remaining Hawaiian land snail fauna. Calls for development of approaches to protect and expand suitable habitat for native Hawaiian snails have reverberated for 3 decades (Solem, Reference Solem1990), but these efforts have primarily focused on limiting the impacts of non-native predators such as Euglandina species (Hadfield & Mountain, Reference Hadfield and Mountain1980; Hadfield et al., Reference Hadfield, Miller and Carwile1993; Meyer et al., Reference Meyer, Yeung, Slapcinsky and Hayes2017), rats (primarily Rattus rattus; Hadfield et al., Reference Hadfield, Miller and Carwile1993), and increasingly other overlooked predatory species such as Oxychilus alliarius (Meyer & Cowie, Reference Meyer and Cowie2010; Curry et al., Reference Curry, Yeung, Hayes, Meyer, Taylor and Cowie2016). Unfortunately, examination of how changing plant assemblages could have influenced snail populations have been sparse. Until recently, ecological information on plant preferences were undocumented for nearly all extant Hawaiian snails. Identification of critical habitat for threatened arboreal snails in dry forests and snails that live in the leaf litter or surface soil has not been attempted.
Although elucidating mechanisms that underlie snail preferences in wet Hawaiian forests still requires further study, concordant patterns among numerous evolutionarily distinct, endemic insular land snail species in wet Hawaiian forests on three islands suggest conservation strategies that could improve preservation of extant native arboreal snails: (1) protect areas with diverse understorey plant assemblages, (2) restore understorey assemblages in degraded areas, and (3) ensure that important plant species (e.g. B. arguta) are a focus of restoration. Many questions remain, however: (1) How have plant distributions and abundances changed and do these changes correspond with changes in arboreal snail distributions and abundances? (2) How do different plant species influence the fitness of arboreal snails? (3) How have changes in plant assemblages interacted with introduced predators to influence arboreal snail distributions and population sizes? (4) What plant restoration efforts could best enhance land snail conservation efforts? We hope this study helps spur further holistic research to explore how plant restoration efforts could benefit not only native snails but also other native Hawaiian invertebrates.
Acknowledgements
We thank Maui Land & Pineapple Co. Inc., Puʻu Kukui Watershed Preserve staff for field and logistical support, and Garrett Ancheta and Kainoa Pestana for their invaluable assistance with field work. Funding was provided by the National Science Foundation to NWY, KAH, and WMM (DEB-1819878), and to NWY and KAH (DEB-1120906, 1656254, 1656231, 1837849, DBI-1902328, DBI-1561774). This is contribution 2020-006 to the Hawaii Biological Survey.
Author contributions
Study design: WMM, NWY, KAH; fieldwork: all authors except JRK; data analysis: LME, CJKK, WMM; writing: WMM, KAH, NWY; figure, supplementary tables: JRK; revision: all authors.
Conflicts of interest
None.
Ethical standards
This study abided by the Oryx guidelines on ethical standards. All collections of snails were under the provisions of state and local permits (FHM11-264, FHM12-294, FHM13-324, FHM15-357, I1053, I1271), in collaboration with Puʻu Kukui Watershed Preserve staff, and with minimization of impacts on remaining populations and their ecosystems.




