Introduction
Leatherback turtles Dermochelys coriacea were categorized as Critically Endangered on the IUCN Red List in 2000 on the basis that they faced ‘an extremely high risk of extinction in the wild in the immediate future’ (Sarti-Martínez, Reference Sarti-Martínez2000). This categorization has since been revised at the regional level because of varying population trends in the different ocean basins (Godfrey & Godley, Reference Godfrey and Godley2008; Seminoff & Shanker, Reference Seminoff and Shanker2008; Wallace et al., Reference Wallace, Tiwari and Girondot2013). An overview suggested the majority of populations in the Atlantic Ocean were stable or increasing, with some subpopulations, such as in the North-west Atlantic, categorized as Least Concern (Dutton et al., Reference Dutton, Dutton, Chaloupka and Boulon2005; Turtle Expert Working Group, 2007; Girondot et al., Reference Girondot, Godfrey, Ponge and Rivalan2007; Wallace et al., Reference Wallace, Tiwari and Girondot2013). However, the Pacific populations were undergoing significant declines (Spotila et al., Reference Spotila, Reina, Steyermark, Plotkin and Paladino2000; Reina et al., Reference Reina, Mayor, Spotila, Piedra and Paladino2002; Seminoff et al., Reference Seminoff, Paladino and Rhodin2007). These differing trends make it necessary to analyse threats and population responses at the regional level. Wallace et al. (Reference Wallace, DiMatteo, Hurley, Finkbeiner, Bolten and Chaloupka2010) defined Regional Management Units as a tool for identifying nesting areas and improving inter-regional understanding of marine turtle nesting distribution.
More information about turtle distribution and reliable estimates of demographic trends by capture–mark–recapture techniques are needed for a better analysis of the status and health of wild populations (Chaloupka & Limpus, Reference Chaloupka and Limpus2001). The size of leatherback turtles varies among populations (Stewart et al., Reference Stewart, Johnson and Godfrey2007), and Atlantic Costa Rican leatherback turtles exhibit greater reproductive output than their eastern Pacific counterparts, with clutch sizes of 80–90 eggs per clutch in the Atlantic and 60–65 eggs in the Pacific (Chacón, Reference Chacón1999; Reina et al., Reference Reina, Mayor, Spotila, Piedra and Paladino2002; Hilterman & Goverse, Reference Hilterman and Goverse2007; Quiñones et al., Reference Quiñones, Patiño-Martinez and Marco2007). Growth rates of adult females after reaching sexual maturity are > 0.2 cm per year in Pacific leatherback turtles (Price et al., Reference Price, Wallace, Reina, Spotila, Paladino, Piedra and Vélez2006). It is estimated this species reaches sexual maturity at 5–30 years (Zug & Parham, Reference Zug and Parham1996; Dutton et al., Reference Dutton, Dutton, Chaloupka and Boulon2005; Jones, Reference Jones2009) and captive animals mature at 7–16 years of age (Jones et al., Reference Jones, Hastings, Bostrom, Pauly and Jones2011). Remigration intervals are shorter and more stable in the Atlantic (2–3 years) than in the Pacific (c. 3.7 years), possibly reflecting greater foraging success in the Atlantic (Reina et al., Reference Reina, Mayor, Spotila, Piedra and Paladino2002; Dutton et al., Reference Dutton, Dutton, Chaloupka and Boulon2005; Girondot et al., Reference Girondot, Godfrey, Ponge and Rivalan2007; Bailey et al., Reference Bailey, Fossette, Bograd, Shillinger, Swithenbank and Georges2012). The annual mortality rate of adult females is estimated to be c. 11% for the Atlantic population of St Croix (Dutton et al., Reference Dutton, Dutton, Chaloupka and Boulon2005) and c. 22% for the Costa Rican Pacific population (Santidrián Tomillo et al., Reference Santidrián Tomillo, Saba, Piedra, Paladino and Spotila2007). Hatching success of leatherback turtles (c. 50%) is low compared to other marine turtles (reviewed by Bell et al., Reference Bell, Spotila, Paladino and Reina2004; Wallace et al., Reference Wallace, Sotherland, Spotila, Reina, Franks and Paladino2004) such as Atlantic green turtles Chelonia mydas (63.5–86.0%) in Tortuguero (De Haro et al., Reference De Haro, Troeng, Harrison, Rees, Frick, Panagopoulou and Williams2008; Segura & Cajade, Reference Segura and Cajade2010), hawksbill turtles Eretmochelys imbricata (78.6%) in the West Indies (Ditmer & Stapleton, Reference Ditmer and Stapleton2012) or loggerhead turtles Caretta caretta (73.4, 55.7 and 70–80%, reported in South Carolina (Caldwell, Reference Caldwell1959), Florida (Witherington, Reference Witherington1986), and Cape Verde (Abella et al., Reference Abella, Marco and López-Jurado2007), respectively).
The main threats to leatherback turtles include poaching, commercial and artisanal fisheries (Morreale et al., Reference Morreale, Standora, Spotila and Paladino1996; Eckert & Sarti Reference Eckert and Sarti1997; Alfaro-Shigueto et al., Reference Alfaro-Shigueto, Dutton, Van Bressem and Mangel2007; Seminoff et al., Reference Seminoff, Paladino and Rhodin2007; Santidrián Tomillo et al., Reference Santidrián Tomillo, Saba, Piedra, Paladino and Spotila2008), and climate change (Saba et al., Reference Saba, Santidrián Tomillo, Reina, Spotila, Musick, Evans and Paladino2007; Reina et al., Reference Reina, Spotila, Paladino and Dunham2009; Santidrián Tomillo et al., Reference Santidrián Tomillo, Saba, Blanco, Stock, Paladino and Spotila2012). However, population responses to threats may differ among ocean basins.
The Caribbean coast of Central America may host the fourth largest nesting population of leatherback turtles (Patiño-Martínez et al., Reference Patiño-Martinez, Marco, Quiñones and Godley2008), after Gabon, French Guiana–Suriname and Trinidad & Tobago (Turtle Expert Working Group, 2007). On the Caribbean coast of Costa Rica the most important leatherback rookeries, with long-term monitoring records, are at Tortuguero, Pacuare Nature Reserve and Gandoca beaches (Troëng et al., Reference Troëng, Chacón and Dick2004; Chacón-Chaverri & Eckert, Reference Chacón-Chaverri and Eckert2007). However, no previous scientific studies have assessed the status of the nesting population at Pacuare Nature Reserve despite the long-term monitoring and conservation programme, established in 1994. Because of the nature of the study and the relatively high nesting levels in Caribbean Costa Rica the Reserve could be an important contributor to the regional assessments of the species in the Caribbean region.
We assessed the importance of Pacuare Nature Reserve as a nesting site for leatherback turtles in the Caribbean by analysing nesting abundance and trends over 18 years, describing some aspects of their nesting ecology, and identifying the main threats and proposing conservation priorities to increase the population.
Study area
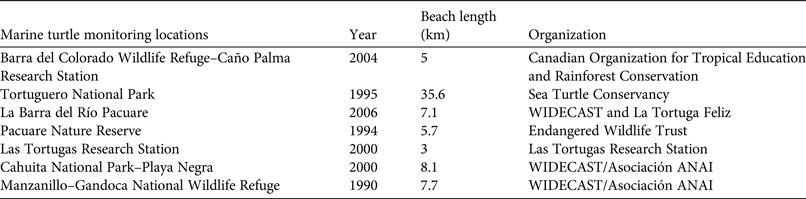
We conducted the study at Pacuare Nature Reserve, 45 km south-east of Tortuguero on the Caribbean coast of Costa Rica (Fig. 1). The mouth of the Pacuare river is 1 km from the northern limit of the Reserve and the Mondonguillo lagoon is located at the southern end of the Reserve. The nesting beach is 5.7 km long. The beaches in this area are high-energy sandy beaches with medium steep slopes. A number of beach monitoring projects have been conducted along this coast (Fig. 1) but long-term monitoring programmes (> 10 years) have been carried out at only five of these sites: Pacuare Nature Reserve, Tortuguero National Park, Las Tortugas Research Station, Manzanillo–Gandoca National Wildlife Refuge and Cahuita National Park–Playa Negra (Table 1). There are ongoing monitoring projects at the first three sites.

Fig. 1 Locations of marine turtle monitoring projects along the Caribbean coast of Costa Rica, Central America.
Table 1 Locations along the Caribbean coast of Costa Rica where marine turtle monitoring was carried out, with the year the projects commenced, the length of beach monitored, and the organizations leading the projects.
Methods
Nesting surveys
Two stations (north and south) were established at the borders of Pacuare Beach to monitor the total length of the beach. Along the coastline, we placed marker posts to divide the area into 100–m intervals, and marked sub-sectors every 25 m from the south (posts 0–57). Night patrols have been conducted during every nesting season (1 March–30 September) since 1994 (with the exception of 1998) along the total length of the beach, to monitor all nesting activities accurately and minimize egg poaching.
Each patrol team monitored the beach for 4 hours, starting from each station at 20.00, 22.00 and 00.00. Each turtle encountered was tagged and measured, and clutches found in risk-prone areas were relocated. Turtles were tagged with Monel 49 tags (National Band & Tag Co., Newport, USA) on both rear flippers. Turtles that bore no evidence of tags, scars, holes or other deformities were considered new recruits to the rookery (Chacón, Reference Chacón1999; Troëng et al., Reference Troëng, Chacón and Dick2004). Since 2001, subcutaneous microchip passive integrated transponder tags have been used (in < 5% of cases annually) in addition to flipper tags. Morning counts were conducted along the beach, to record every turtle track throughout the nesting season and to camouflage nests by flattening the sand to prevent egg poaching and predation by dogs. We estimated the number of nests per km to determine nest density by dividing the mean annual number of nests by the length of the beach.
Biometry and nesting ecology
We estimated mean nesting success as the proportion of nesting activities with oviposition relative to the abandoned attempts. We measured curved carapace length and width to the nearest mm, following Bolten (Reference Bolten, Eckert, Bjorndal, Albreu-Grobois, Donelly, Eckert, Bjorndal, Abreu-Grobois and Donnelly1999). Length was measured along the right side of the central ridge, and width across the widest part of the carapace from the outermost ridges. Before 2000 only the mean values of number of nests, curved carapace length and clutch size were available from annual technical reports (Rodríguez, Reference Rodríguez1994–1999). Biometric data and clutch size of a sample of remigrant females were also recorded and included in the annual reports. We calculated mean clutch size (number of eggs laid per successful nesting event) for this population over the years from 7,131 records. We recorded number of nests observed during female oviposition and without female presence since 2005.
Since 2000 we have marked each nest with sticks and flagging tape, and measured the distances from nests to the north, south and middle sectorial posts. We monitored nests during incubation and emergence, and excavated them 2 days after hatching, or 75 days after eggs were laid. We calculated hatching success as the proportion of eggs that produced hatchlings: H = S/(S + U), where S = number of eggshells and U = number of unhatched eggs. Eggshell fragments ≥ 50% of the egg surface were considered as one hatched egg (Miller, Reference Miller, Eckert, Bjorndal, Abreu-Grobois and Donnelly1999). The total number of hatchlings at Pacuare Nature Reserve was calculated using the mean number of hatchlings estimated per clutch (hatching success multiplied by number of eggs in a clutch) multiplied by the total number of nests recorded. Research assistants were trained every season to use the same methodology.
A variable number of clutches were relocated each season to stable beach zones close to original sites if they were at risk of erosion and inundation and/or poaching.
Remigrant females
Tagged females or those with evidence of having been tagged previously (presence of holes, scars or skin deformations) were considered remigrants. We estimated growth rates and remigration intervals for a subsample of 330 females that were recaptured during a minimum of three nesting seasons over the study period, and used mean curved carapace length (based on at least two measurements) for each female encountered in each season. At the end of each season, errors > 1 cm among several annual measurements were removed before calculating mean error among observers.
We estimated observed and expected remigration intervals (number of years between consecutive nesting seasons) for every remigrant turtle. Observed intervals were calculated by dividing the number of years between the first and last oviposition by the number of seasons the turtle had nested. We calculated expected remigration intervals for a sample of 330 turtles by considering 2 years as a minimum remigration interval. We added one nesting event when inter-nesting intervals were ≥ 4 years. To calculate the interannual variation in the number of clutches laid we calculated the coefficient of variation (CV) from the mean and standard deviation (SD) of nests per female per year (CV = SD/mean; Broderick et al., Reference Broderick, Godley and Hays2001). Finally, we analysed the variability in clutch size over time for 197 neophytes recorded at Pacuare, and for remigrants (n = 330) that laid nests in at least three seasons and twice in a year. We used mean clutch size when more than two ovipositions were recorded in a season. We analysed the correlation between curved carapace length and clutch size for this sample of remigrants.
We used XLSTAT.7.5.2 v. 2.0 (Addinsoft, New York, USA) and STATISTICA v. 7.0 (StatSoft, Inc., Tulsa, USA) to conduct all statistical analyses. Annual numbers of nesting females (remigrants and neophytes) and correlation between female size and clutch size were analysed using linear regression. Alpha was set at 0.05. We used χ2 tests to compare annual fluctuations of nests, nesting success, female size, clutch size, and hatching success over seasons. We used ANOVA to determine clutch size variations between neophytes and remigrants, and observed vs expected remigration intervals.
Results
Nesting population trends
A total of 14,567 clutches were laid during the 18-year study. The highest nesting density occurred in April and May, followed by March and June (Fig. 2). February and August were excluded from the analysis because low numbers of nests were recorded during these months.
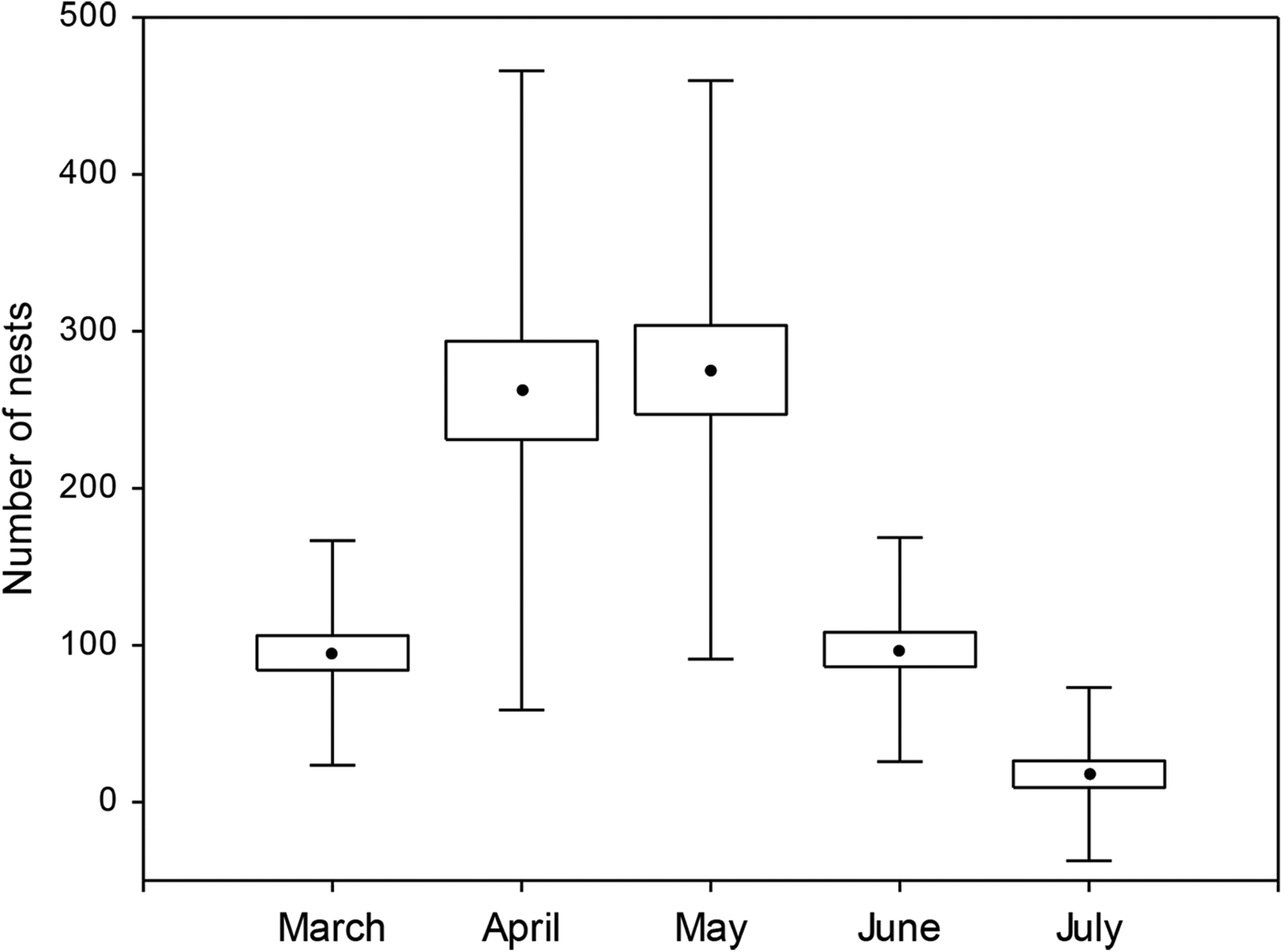
Fig. 2 Box plot of the number of clutches oviposited per month by female leatherback turtles Dermochelys coriacea during 1994–2012. The centre point in the box represents the mean value; the whiskers represent standard deviation.
Overall, there was no significant change in nest numbers over the monitored period (linear regression, r 2 = 0.015, F 1,16 = 0.24, P = 0.63, n = 18; Fig. 3) but in 2012 the greatest number of nests ever (n = 1,206) was recorded at the Reserve.

Fig. 3 Total number of clutches laid and false crawls (abandoned nesting attempts), and nesting females during 1994–2012 (excluding 1998). The numbers above the columns indicate nesting success (%).
We estimated a mean annual nesting density of 142.0 nests km−1 over the duration of the study, with the highest annual nesting density (211.6 nests km−1) occurring in 2012. In 2009 a total of 380 clutches (126.6 nests km−1) were recorded in the northern sectors (0–29) and 789 clutches (263 nests km−1) in the southern sectors (30–60), revealing a trend for higher nesting density in southern compared to northern sectors.
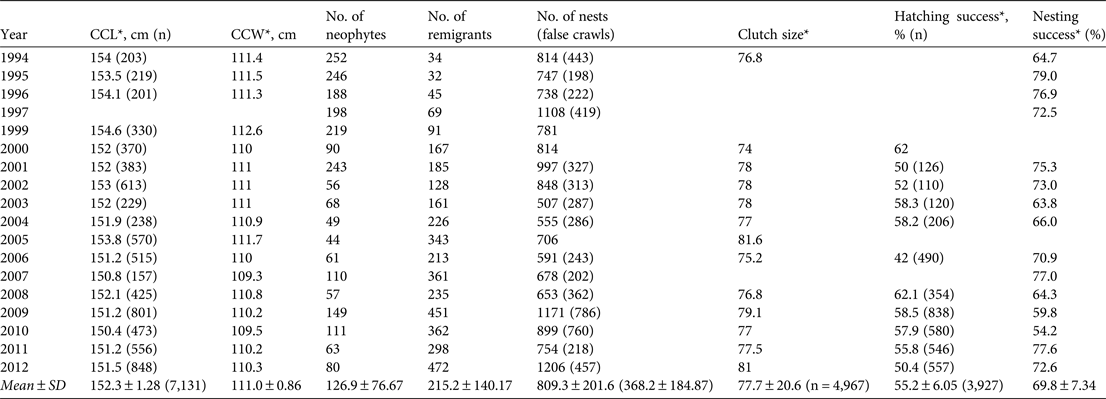
The annual mean number of nesting females was 342 ± SD 118, of which 127 ± SD 77 were neophytes (range 44–252) and 215 ± SD 140 were remigrants (range 32–472; Table 2). Both the annual number of nesting females and the number of remigrant females increased slightly during the study (linear regression, r 2 = 0.40, F 1,17 = 10.75, P = 0.005, n = 18, and r 2 = 0.83, F 1,17 = 80.9, P < 0.001, n = 18, respectively).
Table 2 Summary of biological data recorded during 1994–2012: curved carapace length (CCL), curved carapace width (CCW), number of neophytes, number of remigrants, number of nests and false crawls, clutch size, hatching success and nesting success. Blank cells indicate no data.
* Mean values for 1994–1999 were obtained from technical reports.
During the 18 years of the study 20,090 nesting activities (nests and false crawls) were recorded. Since 2005 a mean of 71.2 ± SD 11.5% of all nests were recorded during female oviposition every season. The mean annual numbers of nests and abandoned nesting attempts were 809.3 ± SD 201.6 (range 507–1,206) and 368.2 ± SD 184.87 (range 198–786), respectively (Table 2). The difference between observed and expected numbers of nests for the hypothesis of non interannual variation was significant (χ2 = 587.03, P < 0.0001), reflecting annual fluctuations. Mean nesting success at Pacuare was 69.8 ± SD 7.3% (range 54.2–79, n = 15; Table 2; Fig. 3). There were no significant differences in percentages of nesting success over the 18 years (χ2 = 10.64, P = 0.71).
Biometry and nesting ecology
The annual mean curved carapace length was 152.3 ± SD 1.28 cm (n = 17 seasons). The smallest and largest turtles recorded in Pacuare Nature Reserve had curved carapace lengths of 138 and 178 cm, respectively. The annual mean curved carapace width was 111.0 ± SD 0.9 cm (n = 17 seasons; Table 2). There was not a significant difference in female size among seasons (χ2 = 16, P = 0.45). The annual mean clutch size over 10 years was 77.7 ± SD 2.1 eggs. We did not find significant differences in mean clutch size among years (χ2 = 13, P = 0.37).
Mean annual hatching success over the study period was 55.2 ± SD 6.0% (Table 2) from a total of 3,927 clutches excavated, and a mean of 47.8 ± SD 25.5% of nests (range 13–83) were excavated per season. The mean hatching success of nests did not change over time (χ2 = 6.47, P = 0.77; Table 2). During 2009–2012 the mean hatching success was 59.4 ± SD 25.8% (range 0–100). We estimated a total of 618,943 hatchlings at the Reserve over the 18-year study period.
Remigrant females
We estimated the annual growth rate of nesting females was 0.28 ± SD 1.0 cm per year (range 0–3.1; n = 330). The mean clutch size for the 330 females was 81.3 eggs, with a mean individual variation of 14.5 eggs per clutch (range 1–55) for all seasons; this mean clutch size was significantly greater than the mean of 74.3 eggs estimated for neophytes (n = 197; ANOVA, F (329,196) = 1.95, P < 0.0001). There was a significant positive correlation between female size and clutch size (linear regression, r 2 = 0.12, F = 37.21, P < 0.05, n = 271; Fig. 4). Approximately 49% of the females (n = 109) showed a variation in clutch size of 0–10 eggs difference among clutches over the years, c. 25% laid clutches with a variation of 11–20 eggs, and 13.8% laid clutches with a variation of 21–30 eggs. For 12% of females there were differences of > 30 eggs between clutches laid in different nesting seasons.
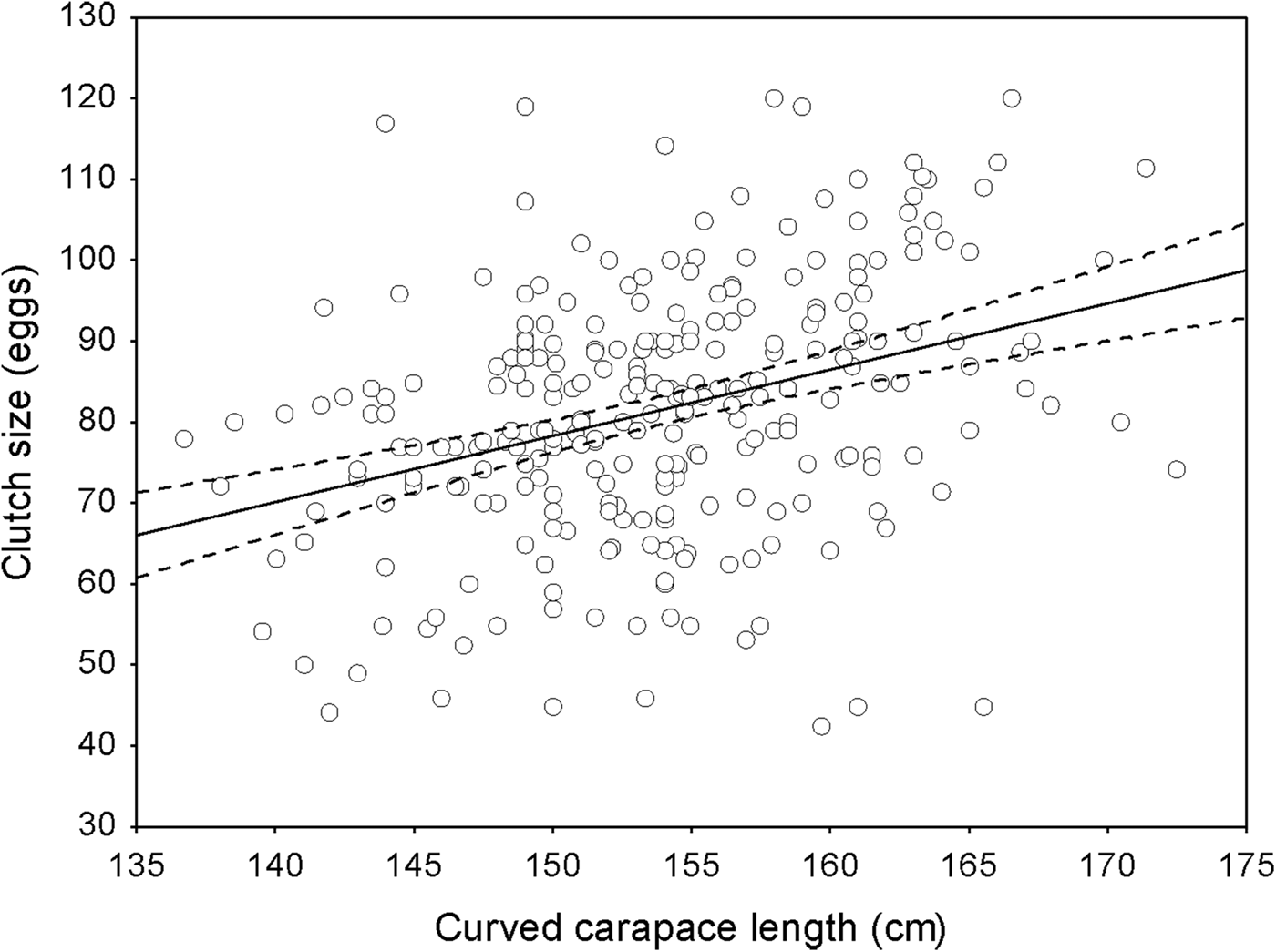
Fig. 4 Relationship between clutch size and female size (curved carapace length). The solid line corresponds to loess regression (n = 330 nesting females) and the dashed lines indicate approximate pointwise 95% confidence intervals.
Finally, the mean remigration interval observed at the Reserve was 2.5 ± SD 1.0 years (range 1–6, n = 330 turtles) and the mean expected remigration interval was 2.1 ± SD 0.6 years (range 1–9, n = 330 turtles). Remigration interval was not related to female size (ANOVA, F = 0.33, P = 0.56). The interannual variation in the total number of observed clutches in any one season was low (CV = 0.32).
Discussion
Nesting trend
The turtle monitoring project at Pacuare Nature Reserve is one of the three oldest established in Caribbean Costa Rica, after Gandoca–Manzanillo National Wildlife Refuge and Tortuguero National Park (Table 1). Despite conservation efforts along the coast there remain extended sections of unmonitored nesting beaches (Fig. 1), where nest poaching and turtle harvesting are common (authors, pers. obs.; Chacón, Reference Chacón1999; Troëng et al., Reference Troëng, Chacón and Dick2004; Chacón-Chaverri & Eckert, Reference Chacón-Chaverri and Eckert2007).
Nesting density in the Reserve is the highest in Central America (142 nests km−1), exceeding the previous estimate of 91.6 nests km−1, based on aerial surveys (Troëng et al., Reference Troëng, Chacón and Dick2004), and the density at Chiriqui beach (128 nests km−1), which was previously considered the most densely nested area (Ordoñez et al., Reference Ordoñez, Troëng, Meylan, Meylan and Ruiz2007). The number of nesting turtles at the Reserve seems to be increasing, with a maximum recorded in 2012. Similar trends have been observed in other Atlantic rookeries, such as St Croix (Dutton et al., Reference Dutton, Dutton, Chaloupka and Boulon2005), French Guiana and Suriname (Girondot et al., Reference Girondot, Godfrey, Ponge and Rivalan2007), and Florida (Stewart et al., Reference Stewart, Sims, Meylan, Witherington, Brost and Crowder2011), as well as for other marine turtle species in the area (Troëng & Rankin, Reference Troëng and Rankin2005; Antworth et al., Reference Antworth, Pike and Stiner2006).
Long-term data are needed for correct interpretation of interannual variations in nesting numbers, imperfect counting of individuals, and the improvement in surveys over the years (Pfaller et al., Reference Pfaller, Bjorndal, Chaloupka, Williams, Frick and Bolten2013). Over the duration of the project a high number of neophytes were recruited to the population. The highest number (n = 243) was recorded in 2001, after which there was no further increase. Movement of female leatherback turtles between nesting beaches is common on the Caribbean coast of Costa Rica, and we have identified turtles tagged at other projects from northern Costa Rica (Caño Palma, Tortuguero; Fig. 1) to northern Panama (Chiriquí beach). A high level of exchange is also suggested by the low nest fidelity at Pacuare (mean 2–3 nests per season) compared to that of other populations (mean c. 7 nests; Reina et al., Reference Reina, Mayor, Spotila, Piedra and Paladino2002).
Biometry and nesting ecology
Turtle sizes were similar to those reported at other nesting sites in the region (reviewed by Thomé et al., 2007). Mean clutch size was similar to those reported for Gandoca and other nesting populations in the Caribbean (Chacón, Reference Chacón1999; Chacón-Chaverri & Eckert, Reference Chacón-Chaverri and Eckert2007; Ordoñez et al., Reference Ordoñez, Troëng, Meylan, Meylan and Ruiz2007) but was slightly lower than in Tortuguero (Leslie et al., Reference Leslie, Penick, Spotila and Paladino1996). Almost half of the females showed low variability in clutch size among years (range 0–10 eggs among clutches). Mean hatching success (55.2%) was higher than that reported for Tortuguero (41.5–46.5%; Troëng et al., Reference Troëng, Harrison, Evans, de Haro and Vargas2007) and did not vary significantly among years.
We found a positive correlation between mean curved carapace length and clutch size of remigrant females, similar to other marine turtle species (Hirth, Reference Hirth1980) but unlike other leatherback turtle populations (Hirth et al., Reference Hirth, Kasu and Mala1993; Reina et al., Reference Reina, Mayor, Spotila, Piedra and Paladino2002). We estimated the mean growth rate of nesting females was 0.28 ± SD 1.0 cm per year, faster than that reported by Price et al. (Reference Price, Wallace, Reina, Spotila, Paladino, Piedra and Vélez2006) for Pacific leatherback turtles. At a growth rate of 0.28 cm per year it would take 143 years for a turtle measuring 138 cm (the smallest nester) to grow to 178 cm (the largest nester). Thus, it seems a reasonable assumption that the variation in female size is caused by size variability at the time they reach maturity, rather than growth after the time of first reproduction.
Observed remigration intervals at Pacuare Nature Reserve were 2.5 years, similar to those recorded by Dutton et al. (Reference Dutton, Dutton, Chaloupka and Boulon2005) in St Croix. Interannual variation in nesting numbers was lower than that recorded in French Guiana, and probably reflects consistent foraging conditions for leatherback turtles in the Atlantic across years (Broderick et al., Reference Broderick, Godley and Hays2001).
Threats and conservation implications
Marine turtles face persistent threats from fishery by-catch (Alfaro-Shigueto et al., Reference Alfaro-Shigueto, Dutton, Van Bressem and Mangel2007; Georges et al., Reference Georges, Billes, Ferraroli, Fossette, Fretey and Gremillet2007), egg poaching (Troëng et al., Reference Troëng, Chacón and Dick2004, Reference Troëng, Harrison, Evans, de Haro and Vargas2007; Ordoñez et al., Reference Ordoñez, Troëng, Meylan, Meylan and Ruiz2007) and tourism (Katselidis et al., Reference Katselidis, Schofield, Stamou, Dimopoulos and Pantis2013). Intensive beach patrolling and the presence of guards reduced poaching of eggs at Pacuare compared to poaching levels before the project was established. The lowest number of nests was recorded in 2003 and nesting levels remained low until 2009, possibly as a result of the high levels of egg poaching that occurred at the site prior to the early 1990s. Although poaching was reduced to c. 1–2% per year after protection started, c. 10% of clutches were still poached in 2008.
Conservation programmes in protected areas can facilitate recovery of marine turtle populations (Revuelta et al., Reference Revuelta, León, Feliz, Godley, Raga and Tomás2012). Nesting population levels at Pacuare Nature Reserve indicate that long-term beach protection may be an effective conservation mechanism, reducing poaching and increasing beach productivity. However, declining trends were reported for Gandoca and Tortuguero in 2004 and 2006, respectively (Chacón-Chaverri & Eckert, Reference Chacón-Chaverri and Eckert2007; Troëng et al., Reference Troëng, Harrison, Evans, de Haro and Vargas2007), and it is possible that turtles shifted from those locations to Pacuare, given the low site fidelity exhibited in this area. To assess population trends and status for the Caribbean Regional Management Unit as a whole, it is necessary to collate information from various research projects. Conservation programmes will continue at Pacuare Nature Reserve and a complete regional analysis will be the future research challenge.
Acknowledgements
We thank the Endangered Wildlife Trust Directors John Denham and Carlos Fernandez for their management of Pacuare Nature Reserve and their support for conservation projects. We also thank the coordinators and assistants at Pacuare Nature Reserve, without whom this project would not have been possible: Stanley Rodríguez, Belinda Dick, Rubén Venegas, David Melero, Scott Handy, Sara Lucas, Isabel Rose and Francisco Ferrer. We thank Pilar Santidrián, David Booth, Graeme Hays and Connie Foss for their comments. Research permits were obtained from the Ministry of Environment and Energy (MINAE) of Costa Rica (R-SINAC-ACLAC-PIME-009-2013).
Biographical sketches
Marga Rivas has been studying marine turtles in the field for over 8 years and has studied the biology of nesting populations of leatherback, green, loggerhead and olive ridley turtles. Carlos Fernández has directed the marine turtle monitoring project carried out at Pacuare Nature Reserve for the past 25 years. Adolfo Marco's research interests focus on marine turtle biology and ecology, and conservation of loggerhead and leatherback turtles.








