Introduction
Non-timber forest products (NTFPs) are no longer regarded as resources of only minor significance; they are now seen to offer potential benefits for rural development and the conservation of forests (Peters et al., Reference Peters, Gentry and Mendelsohn1989; Schwartzman et al., Reference Schwartzman, Moreira and Nepstad2000; Higgins-Zogib et al., Reference Higgins-Zogib, Dudley, Mansourian, Suksuwan, Stolton and Dudley2010). Arnold & Ruíz-Pérez (Reference Arnold and Ruíz-Pérez2001) identified three main strands linking NTFPs to forest conservation: NTFPs contribute to the livelihoods and welfare of people living in and adjacent to forests; commercial harvest of NTFPs adds to the perceived value of the forest and hence the incentive to preserve it; and NTFP exploitation is less destructive than logging and other forest uses and can therefore provide a sound base for sustainable forest management. Bolstered by these premises, authorities in many protected areas permit local communities to harvest nuts, leaves, bark, resin, roots and other plant products, even where hunting and timber extraction are forbidden (Coad et al., Reference Coad, Campbell, Miles and Humphries2008). Some researchers believe traditional practices have ‘inherently assured the sustainable harvesting of NTFPs’ (Ahmad, Reference Ahmad2003) but others have raised concerns that even customary extraction methods can endanger certain species and damage the forest (Ndangalasi et al., Reference Ndangalasi, Bitariho and Dovie2007; Stanley et al., Reference Stanley, Voeks and Short2012).
Tree resins are among the most sought-after NTFPs because they are non-perishable and have a relatively high value per unit weight (Newton et al., Reference Newton, Watkinson and Peres2011). Copals, dammars and other hard resins are used in varnishes, adhesives, paints, plastics and fireworks, whereas the softer, odoriferous oleoresins, such as frankincense, turpentine, copaiba and myrrh, are used for medicinal purposes and in incense (Langenheim, Reference Langenheim2003). Their global economic value is considerable: agarwood oleoresin alone generates annual revenue of USD 6–9 billion (Akter et al., Reference Akter, Islam, Zulkefeli and Khan2013). Overexploitation has resulted in many lucrative species becoming threatened with extinction, however, including most species of agarwood (Aquilaria spp.) and frankincense trees (Boswellia spp.) that have been assessed (IUCN, 2014). Researchers in this field have focused primarily on resin production and trade (e.g. Johnson, Reference Johnson1998; Tadesse et al., Reference Tadesse, Feleke and Eshete2004; Ali et al., Reference Ali, Fadl and Adam2009) rather than on the species’ ecology or the impacts of exploitation.
One of the lesser-known species exploited by the resin industry, the lansan tree Protium attenuatum (Burseraceae), is endemic to the mid-elevation rainforests of the Eastern Caribbean, specifically Dominica, Guadeloupe, Grenada, Martinique, Saint Christopher and Nevis, Saint Lucia, and Saint Vincent and the Grenadines (Howard, Reference Howard1988; Rollet, Reference Rollet2010). Although currently categorized as Data Deficient on the IUCN Red List (WCMC, 1998), more recent research indicates the lansan should be categorized as Endangered. Few lansan trees remain on Martinique and Guadeloupe, and surveys in 2014 failed to find any on Grenada or Saint Vincent. Only Saint Lucia and Dominica are confirmed to hold substantial populations (AP, unpubl. data). In Saint Lucia the tree's aromatic fruits are an important food source for wild animals, including the Vulnerable Saint Lucia amazon Amazona versicolor (Collar et al., Reference Collar, Gonzaga, Krabbe, Madroño Nieto, Naranjo, Parker and Wege1992).
Lansan trees are still abundant in Saint Lucia's rainforests but face increasing pressure from uncontrolled extraction of their aromatic oleoresin (Toussaint, Reference Toussaint2009). Called lansan or l'encens, the white exudate is obtained by slashing the bark, and coagulates and hardens before being scraped off with a knife approximately 2 weeks later. Dried lansan is burned as incense at religious ceremonies, to treat various ailments (e.g. sinus congestion and arthritis), as a mosquito repellent, and to ward off evil spirits (Slane, Reference Slane1987; Broome et al., Reference Broome, Sabir and Carrington2007). In Saint Lucia, lansan is bought by at least 60% of Saint Lucians and by almost every church; it is also exported overseas (Morton, Reference Morton2009; Toussaint, Reference Toussaint2009). Martinique is the largest market, having driven its own stocks to near extinction (Palli, undated; Rollet, Reference Rollet2010). Timber from the lansan tree has almost no economic value because it splits easily and succumbs quickly to insects and decay (Questel, Reference Questel1951; Longwood, Reference Longwood1962).
In Saint Lucia, resin is harvested by full-time tappers, for whom it constitutes the main source of income, and by a growing number of opportunistic harvesters, who do so for extra cash. Most lansan trees are in protected forests (Fig. 1), where entry is prohibited without a permit. Illegal tapping is rife, however, and putatively linked to lack of job opportunities in the banana industry (Toussaint, Reference Toussaint2009). Tappers use a variety of cutting tools and techniques, and each tree may be visited and cut multiple times by different tappers, often leading to disputes when tappers take resin from cuts made by others. Toussaint (Reference Toussaint2009) reported increased tree mortalities and poorer regeneration in heavily exploited areas.

Fig. 1 Locations of forest reserves and major towns in Saint Lucia. The rectangles show the locations of the study sites in Barre de l'Isle and Chassin Forests.
The destruction of lansan trees by tappers posed a dilemma for the Government of Saint Lucia, which has a responsibility to conserve the largest remaining population of lansan trees, notwithstanding the species’ cultural and economic importance and that the tappers who harvest it typically come from the poorest sectors of rural society. There have been calls for lansan to be harvested sustainably (e.g. van Eynde, Reference van Eynde2009; Morton, Reference Morton2009), but can resin be extracted without debilitating the wild stocks?
Here we describe an experimental study to (1) measure the quantity of resin produced from lansan trees of different sizes, using conventional and alternative tapping techniques, (2) evaluate and compare the effects of various tapping methods on the health and survival of lansan trees, and (3) determine whether consumers prefer resin harvested using traditional or alternative tapping techniques. The primary purpose was to guide the development of a sustainable harvesting programme for lansan trees in Saint Lucia. The secondary purpose was to design a methodology for evaluating extraction methods that could potentially be applied to other resin-producing trees.
One notable innovation that we tested was the application of sulphuric acid solution to cuts in the tree's cambium layer. A variety of chemical stimulants, including sulphuric acid, hydrochloric acid and the herbicide Paraquat (Tsoumis, Reference Tsoumis1992; Nair, Reference Nair2003), are used in the extraction of resins and gums from trees but none of these had been tested on lansan trees.
Study area
Saint Lucia (617 km2) is a Small Island Developing State in the Lesser Antilles (Eastern Caribbean), with a population of 165,595 (2010 census). Lansan trees occupy the lower montane forests (Graveson, Reference Graveson2009), mostly within an area of c. 6,975 ha within the protected forest reserves (Daltry, Reference Daltry2009; Toussaint, Reference Toussaint2009). Lower montane forest occurs at 100–680 m altitude in Saint Lucia (Graveson, Reference Graveson2009) and most lansan trees are concentrated at 300–550 m. These are wet, evergreen forests, with annual precipitation of 2,000–4,000 mm (CCA, 1991).
To examine the effects of extracting resin using traditional and alternative tapping methods, two sites were selected in the forest reserves where lansan trees are locally abundant (Fig. 1). (1) The Barre de l'Isle Forest is near the centre of Saint Lucia, at 275–365 m altitude. The survey area was only a few hundred metres from a Forestry Department station and checkpoint, and lansan trees in this area were largely untouched. The study trees were accessed along an existing hiking trail. (2) In Chassin Forest (altitude 160–335 m), in north-east Saint Lucia, lansan trees are harvested illegally by tappers from the village of Chassin. Access to the Chassin study area was via the tappers’ foot-trails, starting from the Chassin Sky Rides, a tourist attraction on the edge of the forest reserve.
Methods
Barre de l'Isle Forest
This forest was used for an experimental study of the impacts of various tapping methods on resin yield and the health and survival of trees. We selected 298 healthy lansan trees in four size classes: small (15.0–19.9 cm diameter at breast height, DBH), medium (20.0–24.9 cm DBH), large (25.0–29.9 cm DBH) and very large (≥ 30.0 cm DBH). None had been tapped previously, judging from the absence of scars on their trunks. Controls (X) and nine tapping methods (A–I, Table 1) were assigned randomly to trees within each size class. Only very large trees were subjected to wide-faced tapping (G), as this was considered too invasive for smaller trees. Groups A–G were visited and tapped every 2 weeks for 17 months, during October 2010–March 2012. Groups H and I were tapped every 2 weeks during March 2011–March 2012 (these were added following a review of the preliminary findings from these techniques, using 20 large and very large trees that had not been tapped previously). Every tree was labelled with a numbered tag and had one treatment assigned to it throughout the study. Visits were conducted every 2 weeks, the interval used by local tappers to allow resin to harden fully before collection.
Table 1 Tapping treatments applied to lansan trees Protium attenuatum in the Barre de l'Isle and Chassin Forests of Saint Lucia (Fig. 1), with description and sample size.

Of the treatments tested, B, C, H, and especially A and E are extraction methods traditionally used by lansan collectors in Saint Lucia. Using a resin stimulant (30% sulphuric acid solution in treatments D, F and G, 5% sulphuric acid solution in treatment I) was a new innovation.
On every 2-weekly visit to every tree all resin that had coagulated around the cut was scraped carefully into an individual clear plastic bag, dried and weighed. To determine how tapping treatments affect tree growth and condition, a suite of variables were recorded from all of the Barre de l'Isle study trees before, during and after the experiment (Table 2).
Table 2 Variables measured as indicators of tree condition. In Barre de l'Isle measurements were made for every study tree at the start of the tapping experiment (October 2010 for treatment groups X and A–G; May 2011 for treatment groups H and I), at several intervals during the experiment (October 2010–March 2012), and in July 2013 (16 months after the experiment ended). In Chassin measurements were recorded in February 2011, August 2011, March 2012 and July–August 2013. Where possible, variables were recorded by AT and AP for consistency.

Chassin Forest
In Chassin Forest we assessed the impacts of customary protocols followed by local tappers, using untapped lansan trees at the site as experimental controls. At random we selected 38 trees with signs of recent cuts, and 36 trees that had never been tapped (unmarked), in the four size classes (small, medium, large and very large; Table 1). Many of the tapped trees showed signs of having been tapped multiple times prior to the study, and those that appeared unhealthy or broken were rejected. Trees were inspected every 2 weeks for 13 months, during February 2011–March 2012, with a final appraisal in August 2013. Their condition was evaluated using the same variables as the first site (Table 2). We did not collect resin from the tapped trees, to avoid discouraging the dozen or more tappers from operating in this area. The tappers were not informed the study was taking place nor advised to change their usual routines. The lansan trees were not tagged, to avoid revealing which ones were under observation.
Consumer survey
A simple blind experiment was carried out to gauge whether experienced consumers could detect any differences between the resin produced with or without 30% sulphuric acid stimulant. Two samples of lansan from Barre de l'Isle (one produced without stimulant, pooled from treatments A, B, C and E, and one with stimulant, pooled from treatments D, F and G) were given to seven Roman Catholic churches in seven parishes. The priests, who were accustomed to using lansan as incense, were not informed which sample was which. They were asked to judge the samples based on ease of burning, how well the smoke rose, scent and overall quality, scoring each variable on a scale from 1 (very poor) to 5 (very good).
Data analysis
Descriptive statistics and Mann–Whitney tests, Pearson's tests and Wilcoxon signed rank tests were conducted using XLSTAT v. 2013.1.01 (Addinsoft, Paris, France). To evaluate possible causes of tree mortality at the Barre de l'Isle site, partial Mantel matrix association tests were conducted using software developed by Roger S. Thorpe, to partial out the effects of collinearity between variables (Legendre & Fortin, Reference Legendre and Fortin1989). Trees were organized into 35 groups according to treatment (e.g. X, A, B, C) and girth (DBH), and mortality was measured as the proportion of each group that had died by July 2013. Seven variables putatively associated with mortality (DBH, tapping treatment, lansan yield, termites, cavities, cankers, crown health; Table 2) were tested simultaneously with a partial Mantel test using 10,000 randomizations. Statistical significance was at P < 0.05 after applying a sequential Bonferroni correction (Rice, Reference Rice1989), although this may be overly cautious (Moran, Reference Moran2003).
Results
At the start of the study, mean DBH of the 298 trees in the Barre de l'Isle Forest was 26.15 ± SD 7.84 cm (range 15.0–59.8 cm) and mean DBH of the 74 trees in the Chassin Forest was 26.90 ± SD 10.02 cm (range 16.0–64.0 cm). Local tappers in the Chassin Forest used a variety of techniques, including narrow-face tapping and multiple horizontal or diagonal cuts, but did not apply any stimulant. Individual trees were often found cut using two or more cutting methods, suggesting more than one tapper had worked on them.
Quantity of resin produced (Barre de l'Isle only)
Data from all tapped trees in the Barre de l'Isle Forest revealed a highly significant correlation between quantity of lansan produced and tree size (DBH) (Pearson's correlation coefficient (r) = 0.298, P < 0.0001). Across all treatment classes very large trees (≥ 30.0 cm DBH) typically produced > 60% more lansan than trees in the smallest size category (15.0–19.9 cm DBH). Irrespective of tree size or treatment, lansan yields increased over time (Fig. 2).

Fig. 2 Variation in mean resin yield (dry mass) across all treatment groups in the Barre de l'Isle Forest over time. Trees were tapped every 2 weeks from October 2010 for treatments A–G (n = 240) and from May 2011 for treatments H and I (n = 20).
Resin production was also influenced by cutting method (Fig. 3). Trees produced more lansan when cuts were reopened instead of making new cuts elsewhere on the trunk, and treatments involving sulphuric acid solution consistently produced the largest yields. Across all size classes, treatment D (with 30% sulphuric acid) produced on average 134% more resin per tree per visit (n = 38, mean lansan yield = 5.45 ± SD 3.60 g) than the stimulant-free treatment B (n = 41, mean yield = 2.33 ± SD 2.06 g, U = 253.5, P < 0.0001). Even large trees subjected to treatment B (25–41 cm DBH, mean yield = 2.81 ± SD 2.63 g) could not match the productivity of smaller trees subjected to treatment D (15–24.9 cm DBH, mean yield = 4.40 ± SD 2.30 g, U = 92, P = 0.010). Similarly, among the trees tapped using a narrow-faced cut, 84% more resin was produced by applying 30% sulphuric acid (treatment F, mean yield = 6.52 ± SD 4.20 g) than when no stimulant was used (treatment E, mean yield = 3.54 ± SD 2.00 g, U = 328, P < 0.0001). Applying a weaker solution of 5% sulphuric acid was also effective, with treatment I producing 58% more resin than treatment B from trees in the same size categories.
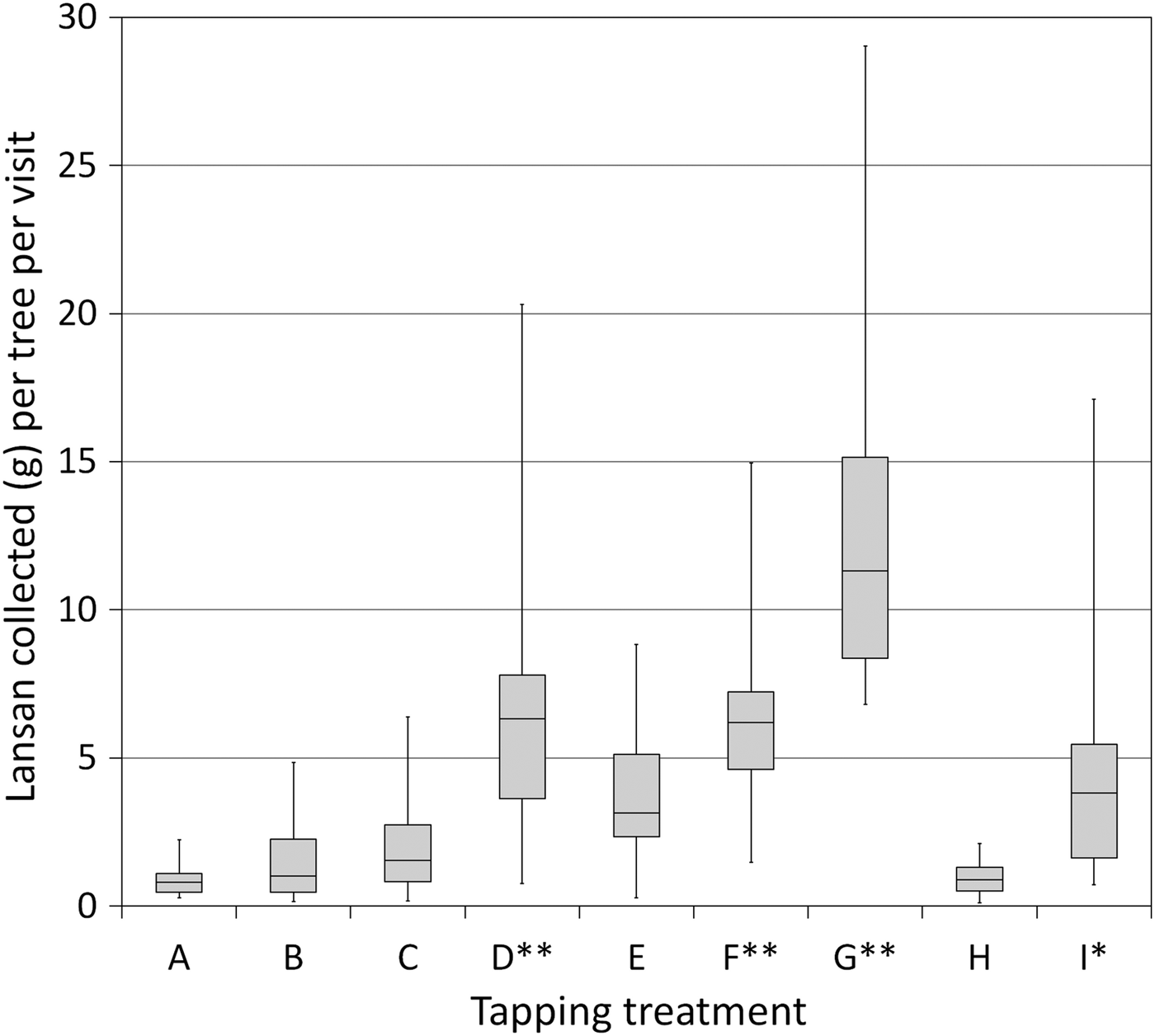
Fig. 3 Mean resin yield (dry mass) from various tapping methods, over 16 2-weekly harvests, from trees ≥ 25 cm DBH. Tapping treatments follow Table 1 (**, treatments using 30% sulphuric acid; *, treatments using 5% sulphuric acid). The upper and lower portions of the box plot are the third and first quartiles, respectively, and the horizontal line is the median. Whiskers indicate the minimum and maximum values. Treatment G, the most intrusive method, was applied to very large trees only (≥ 30.0 cm DBH).
Tree growth and condition
In the Barre de l'Isle, growth rates tended to be faster among smaller, younger trees, with a significant inverse correlation between a tree's DBH at the start of the study and its percentage increase in girth by July 2013 (n = 310, Pearson's correlation coefficient (r) = −0.186, P < 0.001). Control trees exhibited the fastest growth rates during October 2010–March 2012, when the other trees were tapped every 2 weeks (Fig. 4). However, when all of the study trees were re-measured 16 months after tapping ended, treatment categories that had shown poor growth during the experiment exhibited a strong growth spurt (Fig. 4). By July 2013, treatments B and F almost matched the control trees in net increase in girth.

Fig. 4 Relationship between tapping method and tree growth during and after the experiment. Mean net growth from the start of the study to January 2012 (shortly before tapping ended), and mean net growth to July 2013 (16 months after tapping ended). Tree growth is presented as the percentage increase in DBH from the start of the study (October 2010 for control trees X and treatments A–G, and May 2011 for treatments H and I). Tapping treatments follow Table 1 (**, treatments using 30% sulphuric acid; *, treatments using 5% sulphuric acid). Only data from the largest tree size categories are shown (trees ≥ 25 cm DBH when the study began). For comparison, trees in the same size categories in the Chassin study site grew by 4.73% (control trees, X) and 0.93% (tapped trees, T) during February 2011–July/August 2013.
Both during and after the tapping study there was no evidence that sulphuric acid affected growth rates. The growth of trees tapped using the same cutting method with 30% sulphuric acid (treatment B, mean = 4.34 ± SD 3.14%), for example, did not differ significantly from trees tapped without the acid (treatment D, mean = 4.37 ± SD 3.62%, U = 790, P = 0.914).
In Chassin, tree girth was measured in February 2011 and August 2013. Control trees grew almost twice as fast as the tapped trees and there was no significant difference between their percentage increase in girth (n = 36, mean = 3.42 ± SD 3.29%) and that of control trees in Barre de l'Isle (n = 40, mean = 3.63 ± SD 2.81%, U = 789.5, P = 0.473). However, the tapped trees in Chassin showed significantly poorer growth (n = 38, mean = 1.82 ± SD 2.47%) than trees tapped using narrow-face tapping in Barre de l'Isle (treatment E: n = 38, mean = 4.68 ± SD 5.18%, U = 1,147, P < 0.0001) during the same period. Treatment group E was chosen for comparison because many trees in Chassin exhibited signs of narrow-face tapping.
Ten months into the experimental study in Barre de l'Isle the untapped trees (X) and treatment groups H and I had the best quality crowns, with mean scores of 4.0 or higher. No groups in Barre de l'Isle had mean scores < 3.6, however, indicating most trees were in reasonable condition. Trees used by tappers in Chassin had thinner crowns by 2013 (n = 38, mean score = 3.48 ± SD 0.85) but were not in significantly worse condition than the control trees in the same area (n = 36, mean score = 3.67 ± SD 0.78, U = 393, P = 0.398).
The trunks of many individuals in Barre de l'Isle, including the control trees, had at least one termite trail at the beginning of the study, but there was no net change in termite abundance by the end. Fungal infection was detected only rarely among the Barre de l'Isle trees. By the end of the study in Chassin, however, tapped trees had on average 3.5 times more termite trails (mean = 0.59 ± SD 0.60 trails) than the control trees (mean = 0.18 ± SD 0.37 trails, U = 817, P = 0.003) and significantly more fungal infections (mean = 0.242 ± SD 0.44) than the controls (mean = 0.03 ± SD 0.17, U = 721.5, P = 0.009).
Among other indicators of tree health (Table 1), we observed a general increase in the number of cavities during the study. The Barre de l'Isle trees subjected to wide-faced tapping (G) exhibited the highest frequency, with a mean net increase of 0.56 cavities per tree during October 2010–July 2013, whereas the control group had a mean net increase of only 0.06 cavities. In Chassin, tapped trees had approximately four times more cavities (mean = 0.42 ± SD 0.64) than the control trees at the same site (mean = 0.09 ± SD 0.39, U = 601.5, P = 0.005), and many more cavities than any of the Barre de l'Isle trees (Fig. 5).

Fig. 5 Relationship between tapping method and the mean numbers of cankers (protruding by ≥ 2 cm), termite trails and cavities by July/August 2013 (16 months after the tapping experiment ended in Barre de l'Isle). Tapping treatments follow Table 1 (**, treatments using 30% sulphuric acid; *, treatments using 5% sulphuric acid). Only data from the largest tree size category (≥ 25 cm DBH) are shown.
There was no clear relationship between treatment and canker development among the Barre de l'Isle trees (Fig. 5) but the tapped trees in Chassin bore more than twice as many cankers (mean = 7.75 ± SD 5.31 cankers per tree) as non-tapped trees (mean = 3.05 ± SD 4.73), and over seven times more cankers than any of the Barre de l'Isle groups (Fig. 5). During the study the Chassin trees had higher rates of developing new cankers (tapped trees: mean net increase of 0.38 cankers per tree per month; control trees: mean net increase of 0.11 cankers per tree per month) than any treatment groups in Barre de l'Isle.
Applying sulphuric acid solution had no perceptible effect on tree condition at the Barre de l'Isle site. Trees tapped with 30% sulphuric acid stimulant (D and F; n = 76) and without (treatments A, B, C and E; n = 154) did not differ significantly in growth rates (U = 5,774, P = 0.871), crown form (U = 5,578, P = 0.553), cankers (U = 5,713, P = 0.745), cavities (U = 5,733, P = 0.517), termites (U = 6,409, P = 0.177) or fungi (U = 5,769, P = 0.509). The only significant difference was that sulphuric acid treatments resulted in significantly more resin (U = 1,839, P < 0.0001).
Tree mortality
In November 2010, shortly after this study began, 12 study trees at the Barre de l'Isle site died during Hurricane Tomas and were replaced with 12 more trees in the same vicinity. Since then 29 other trees in the study group were found dead or broken. These were distributed evenly across the various treatment groups, and three deaths were recorded in the control group. Our data do not reveal any clear relationship between tree mortality and the treatments received but the 20 trees that died or snapped were significantly smaller (mean DBH = 21.31 ± SD 5.24 cm) than the trees that survived (mean DBH = 26.48 ± SD 7.89 cm, U = 4,065, P < 0.003). A partial Mantel test found no significant association between mortality rates and the treatment used, the quantity of lansan produced, or the growth rates or frequency of cankers or cavities, but there was a highly significant association with the number of termite trails on the trunk (P = 0.0012).
In Chassin Forest, trees used by local tappers had a significantly higher rate of mortality (mean mortality rate = 0.15 ± SD 0.37) than the untouched trees between the start of the study and August 2013 (mean = 0.00, U = 810, P = 0.015). The trees that died did not differ significantly from the surviving trees in Chassin in terms of DBH or frequency of termites, cavities, fungi or cankers.
Consumer survey
Seven churches in the towns of Babonneau, La-Resource, Desruisseaux, Gros-Islet, La Clery, Micoud and Anse La Raye carried out blind tests of lansan that had been harvested using 30% sulphuric acid and lansan harvested without any stimulant. The respondents expressed a high level of satisfaction with both samples, and no significant differences were detected in their perceptions of the resin's burning characteristics, smoke, scent or overall quality (Table 3). All but one affirmed they would be willing to pay more for good-quality lansan.
Table 3 Consumer scores for resin produced with and without stimulant, with Wilcoxon V and statistical significance. These are the mean scores from seven churches in Saint Lucia, each of which was subjected to a blind test of two samples of lansan, one produced without stimulant and one produced using 30% sulphuric acid. Variables were scored by the priests on a scale of 1–5, with 5 being the most satisfactory. None of the tests was statistically significant at P < 0.05.

Discussion
Identifying more productive and sustainable methods of extracting resin
By subjecting 372 trees to more than a dozen treatments, the field study provided useful insights into the ecology of lansan trees and their response to tapping. Resin yields were increased by tapping the same individuals repeatedly, tapping larger trees, reopening cuts instead of making new ones elsewhere on the trunk, using narrow-faced or broad-faced tapping and, most strikingly, applying a solution of sulphuric acid. Even the weakest (5%) solution of sulphuric acid boosted resin production significantly.
In other parts of the world sulphuric acid is commonly used as a stimulant for increasing commercial resin production (e.g. spraying a 50% solution of sulphuric acid on fresh cuts on Pinus palustris raises resin production by 31–36% by increasing resin flux and extending the extrusion period; Panda, Reference Panda2008). Despite using weaker concentrations of sulphuric acid, our study of P. attenuatum showed even more striking results, with resin yields increased by 134% with 30% sulphuric acid solution and by 58% with 5% sulphuric acid. Instead of spraying the exposed cut, however, many commercial resin collectors apply pastes containing sulphuric acid and 2-chloroethylphosphonic acid (CEPA; Reynolds & Kossuth, Reference Reynolds and Kossuth1984). Rodrigues et al. (Reference Rodrigues, Azevedo, Sobreiro, Pelissari and Fett-Neto2008) doubled resin yields from Pinus elliottii by using a commercial paste of 20% sulphuric acid and 3.5% CEPA but remarked on the high cost of CEPA. It would be interesting to test commercial pastes and other stimulants on lansan trees but our findings suggest a weak solution (5–10%) of sulphuric acid alone has a sufficiently potent effect. It is also available locally and is affordable.
Our experiment in the Barre de l'Isle Forest confirmed lansan trees can maintain such enhanced yields for at least 17 months of continuous exploitation. Although even the mildest forms of tapping tend to slow tree growth, promote canker development and may result in a poorer crown, tree growth and condition were unaffected by the sulphuric acid solution. The most damaging methods tested entailed deeper cuts, narrow-faced tapping and wide-faced tapping, which are common practices among local tappers but tend to create cavities in the heartwood. After our tapping experiment ended, the lansan trees demonstrated an extraordinary spurt in growth, which suggests factoring in rest periods could be an effective way of enabling lansan trees to cope with exploitation.
Despite our efforts to replicate methods used by tappers in Saint Lucia, the trees at the Barre de l'Isle experimental site remained in better condition than those in Chassin Forest. Trees exploited by local tappers using their customary methods exhibited high rates of decay, with more termites, fungi, cankers and cavities than the non-tapped trees, and relatively high mortality rates. Despite selecting trees that appeared healthy at the start of the study, six of the 38 tapped trees had died by August 2013, a mortality rate of 6.3% per year. When trunks snapped, this usually occurred around the part of the trunk that had been tapped. Unexpectedly, even the untapped (control) trees in Chassin exhibited high frequencies of cankers, which may indicate infectious fungi or bacteria spreading from the many severely tapped and dying trees in this area. Thus, we infer poor tapping practices endanger not only the tapped trees but the untapped trees around them.
These findings suggest that P. attenuatum are fragile trees that can be debilitated rapidly and killed by intensive tapping. The customary strategies employed by tappers in Saint Lucia are damaging, which is perhaps a ‘tragedy of the commons’ (Hardin, Reference Hardin1968). Because tappers enter forest reserves illegally and lack individual rights over the trees it is in their interest to extract as much resin as quickly as possible on every visit, even from trees that are obviously immature or sick. With more Saint Lucians turning to the forests for income, lansan trees are in danger of becoming locally extinct.
Our findings from Barre de l'Isle show that with the aid of a stimulant tappers can harvest more lansan by using fewer cuts, thereby presenting less risk to the trees. To put our findings in context, if 1,000 large lansan trees (> 20 cm DBH) were tapped every 2 weeks for 12 months using the standard same-cut method with 30% sulphuric acid (treatment D) they would yield at least 152 kg pure resin (dry mass), which could be sold raw for at least USD 2,460. Without stimulant the same 1,000 trees would yield on average only 49 kg (USD 790). These are conservative estimates of price because lansan is sold locally by volume rather than mass, and bags of resin are often bulked out with bark. Pure lansan should command higher prices and there is scope to add more value by processing “Saint Lucia frankincense” into incense sticks, soaps and other merchandise for local people, churches and tourists. We lack accurate measures of the quantity of resin collected illegally by tappers in Chassin and other forest reserves, but visual inspections of tapped trees suggest they do not typically generate as much resin as we achieved using the stimulant. Unlike Protium resin harvesters in South America (Plowden, Reference Plowden2001), Saint Lucian tappers are not competing for the resin with bees or other wild animals.
The prospect of each tapper locating and harvesting 1,000 or more mature trees sustainably is realistic given the locally high density of lansan trees in Saint Lucia. Forest plot data from Graveson (Reference Graveson2009) indicate a mean density of c. 50 lansan trees (individuals ≥ 5 cm DBH) per ha in the lower montane rainforest. Using a larger number of plots, Toussaint (Reference Toussaint2009) found a mean of 22.33 stems of lansan trees ≥ 10 cm DBH per ha in the forest reserves. Extrapolating from these data gives an estimated total of 312,000 lansan trees ≥ 10 cm DBH, of which c. 100,000–125,000 are ≥ 20 cm DBH (our conservatively recommended minimum threshold for tapping, see below) in the forest reserve.
A new framework for harvesting lansan sustainably
Our discovery that resin can be harvested using a method that is both more productive and less damaging to the trees than customary practices is an important breakthrough. Drawing on our findings and consultations with forestry officials, tappers and traders, a national management plan for Saint Lucia's lansan trees has been developed (Forestry Department, 2014). This sets out a new framework and protocols to conserve the wild lansan trees while also enabling people to benefit from collecting, trading and using their resin. The plan entails licensing tappers under the Forest Act and the 2008 Forest (Timber and Non Timber Products) Regulations and granting individual rights to harvest lansan in designated areas of the forest reserves. Every tapper will have harvesting rights in a demarcated area of forest containing a high density of mature lansan trees of harvestable size (≥ 20 cm DBH). Tappers must use only the standard same-cut method of tapping, to minimize risk to the trees, and will be trained and provided with ready-mixed 5–10% solution of sulphuric acid to achieve high yields (30% sulphuric acid is more potent but also more hazardous for tappers to transport and use).
The new management strategy incorporates a number of safeguards for the lansan tree population, including routine monitoring of the lansan forest coupes by the Forestry Department and prohibiting tapping trees < 20 cm DBH, thereby allowing smaller mature trees to grow and reproduce without interference (flowering and fruiting have been recorded in lansan trees as small as 4 cm DBH; Rollet, Reference Rollet2010; Forestry Department, 2014). Another precaution is to rotate the coupes to allow trees to recuperate after being tapped for 1 or 2 years. We found that even after 17 months of tapping, most lansan trees recover quickly and make up for lost growth. Finally, to further bolster the country's wild stocks, the Forestry Department has begun cultivating lansan trees in its nurseries for use in reforestation and slope stabilization projects.
The management plan is already being put into action with a pilot group of tappers from Chassin and Dennery villages. The more professional tappers genuinely welcome better regulation of their industry:
Many tappers have expressed strong concern about conservation of the tree and observed what they considered unscrupulous harvesters who do not care about the health of the trees … They strongly expressed the need for the issuance of licenses to those harvesters who have a vested interest in lansan and who are concerned with its conservation. They also expressed the need for enforcement of laws to protect the forest resource and stiffer penalties for unscrupulous harvesters. (Toussaint, Reference Toussaint2009)
By granting them their own coupes and legitimizing their livelihoods, tappers will feel more empowered to look after the forests and will be willing to assist forestry staff by reporting unlicensed tappers and other illegal activities. As the licensing scheme is introduced nationwide an outreach campaign will urge lansan traders, churches and other consumers to buy incense from licensed tappers only. Preliminary discussions indicate traders and churches are willing to comply.
A similar strategy to support livelihoods and forest conservation could feasibly be adopted by tappers and regulatory authorities in other lansan tree range states. Forestry officers in Dominica, Martinique and Guadeloupe have expressed interest in this project, and study tours to Saint Lucia are planned for 2015. Comparable efforts are underway in other countries to develop sustainable resin programmes for other tree species, to support local livelihoods, for example in Guatemala (Neels, Reference Neels2000), the Philippines (Ella, Reference Ella2003), Cambodia (Tola & McKenney, Reference Tola and McKenney2003; Neang, Reference Neang2009), India (Nair, Reference Nair2003) and Ethiopia (Lemenih & Kassa, Reference Lemenih and Kassa2011). Although most programmes have traditional extraction methods at their core, our study illustrates the danger of assuming these are the most appropriate methods for achieving high yields or low impacts (Peters, Reference Peters1994). Adapting our experimental approach of testing and evaluating alternative methods, coupled with consulting local tappers and buyers, could help other projects discover better ways of extracting tree resin, even from species unrelated to P. attenuatum.
To conclude, Saint Lucia now has good prospects of halting the destruction of lansan trees, meeting the demands of churches and other consumers, and ensuring its tappers and traders continue to profit from this important resource. The new management framework (Forestry Department, 2014) has a number of advantages, including the following: lansan trees are still locally abundant; most of their range is protected and under no other threat; a regulatory system already exists for licensing collection of forest products; tappers are willing to engage in improving and legalizing their industry; and resin can be harvested successfully without debilitating wild trees. This satisfies many of the criteria identified by Newton (Reference Newton2008) for achieving tree conservation through sustainable use. As with any new management regime for a globally threatened species, however, it is important to monitor its implementation and outcomes, and to be prepared to make adjustments as new information arises.
Acknowledgements
For assistance with data collection we thank colleagues from the Forestry Department, including Aloysius Charles, Sean Francois, Lenn Isidore, Odetta James, Marthas Peter, Virginie Sealys, Margaret Severin and Vincent Ernest, and volunteers contracted by Durrell Wildlife Conservation Trust, including Imogene Aylwin, Michael Ball, Benjamin Barca, Tristan Chan, Nick Condie, Karin Crofts, Carolla Dalmeier, Twyla Holland, Barbara Schaeffer, April Tyror, Nathan Wood and Robert Williams. We also thank the churches that participated in the consumer survey. This research was supported financially by the Global Trees Campaign, the Franklinia Foundation and the Flagship Species Fund. We thank two anonymous reviewers for constructive comments.
Biographical sketches
Jennifer Daltry has spent more than 20 years developing biodiversity conservation programmes with NGOs and governments in the Caribbean, tropical Asia and Eurasia, often focusing on lesser known globally threatened species. Alfred Prospere and Adams Toussaint are foresters with keen interests in the conservation and sustainable use of Saint Lucia's rich biodiversity. Jana Gengelbach is a graduate in International Forest Ecosystem Management and takes great interest in natural resource management and public participation. Matthew Morton has worked on wildlife research and conservation projects with in-country partners in the Eastern Caribbean since 1993 and has been based in Saint Lucia for over 12 years.










