Introduction
Successful environmental interventions are often characterized by having clearly defined objectives within a simple institutional framework, such as small-scale woodland creation (e.g. Crabtree et al., Reference Crabtree, Bayfield, Chalmers and MacMillan1997; MacMillan et al., Reference MacMillan, Harley and Morrison1998). Success stories tend to be relatively straightforward in terms of institutional design and decision-making structure, and are often easy to monitor and assess in terms of their expenditure and the attainment of objectives. Larger scale projects that cross national boundaries or involve a diversity of stakeholders, such as global wildlife trade bans (Ross, Reference Ross1998) or climate change initiatives such as reduced emissions from deforestation and forest degradation (REDD), are politically and institutionally more complex and diffuse and are prone to failure or stagnation.
There is now considerable interest in reviewing institutionally complex projects to identify general shortcomings and recommendations for better practices (Balmford & Cowling, Reference Balmford and Cowling2006; Manolis et al., Reference Manolis, Chan, Finkelstein, Stephens, Nelson, Grant and Dombeck2009; Black et al., Reference Black, Groombridge and Jones2011; Martin et al., Reference Martin, Nally, Burbidge, Arnall, Garnett and Hayward2012), and it is recognized that factors such as strong institutional support, stakeholder buy-in, and effective leadership are critical to their success (Clark et al., Reference Clark, Reading and Clarke1994; Salafsky et al., Reference Salafsky, Margoluis, Redford and Robinson2002). Furthermore, it has been argued that overt and subtle political considerations can often trump robust scientific evidence in multi-actor and multistage decision making in the conservation arena (Kørnøv & Thissen, Reference Kørnøv and Thissen2000).
We use an innovative temporal–analytic framework that integrates spatial connectivity modelling and a systematic approach to decision analysis to investigate the institutional failures that may lead to the imminent local extinction of a tiger Panthera tigris population in the western Terai Arc Landscape of India, a globally important Tiger Conservation Landscape (Sanderson et al., Reference Sanderson, Forrest, Loucks, Ginsberg, Dinerstein and Seidensticker2006). Tiger conservation is increasingly seen as a global project, with pan-national collaboration anticipated to double tiger numbers by 2022 under the Global Tiger Recovery Program, and we aim to inform the international community about the challenges such initiatives will face.
In this case study we focus on the failure to restore the Chilla–Motichur wildlife corridor, which has deteriorated over the years to the point where, in the western section of the landscape, the tiger population has declined to two female tigers and is no longer viable (Harihar & Pandav, Reference Harihar and Pandav2012). To identify the socio-political factors that imperil this population, we present evidence to demonstrate the decline in tigers and connectivity across the corridor, we review the recommendations provided over the years to mitigate loss in connectivity, and we analyse the decision process involved in implementing these recommendations. We highlight the lessons learnt from this exercise, to guide conservation initiatives aiming to recover tiger populations or similarly threatened species elsewhere.
Study area
The western Terai Arc Landscape, in addition to supporting one of the highest densities of tigers across its range, presents one of the best opportunities to significantly increase tiger populations in India, with an estimated population increase of 68% forecast under appropriate management (Harihar et al., Reference Harihar, Pandav and MacMillan2014). Spanning the Yamuna river in the west to the Gola river in the east, the western Terai Arc Landscape is now split into two disjunct units, referred to as Tiger Habitat Blocks I and II (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004), with poor connectivity as a result of the deterioration of the Chilla–Motichur corridor, which covers c. 3 km2 along the banks of the river Ganges between the Chilla (eastern banks) and Motichur forest ranges (western banks) of Rajaji Tiger Reserve (Fig. 1). Identified in the early 1980s as a tenuous link for the movement of elephants Elephas maximus (Saxena, Reference Saxena1986), it was also promoted as a critical corridor for tigers in subsequent years (Johnsingh, Reference Johnsingh2001).

Fig. 1 (a) Location of the Chilla–Mothichur corridor between Tiger Habitat Blocks I and II in the western Terai Arc Landscape of India, (b) the eastern and western parts of Rajaji Tiger Reserve and (c) details of the settlements and infrastructure in the corridor.
The historical deterioration of connectivity across the corridor may be traced to (1) the expansion of townships (Haridwar and Rishikesh), (2) the resettlement of people displaced by the construction of the Tehri dam and evacuees from landslide-prone areas into several new townships (Khand Gaon I, II & III and Gangabhogpur), (3) the construction of a hydropower canal on the eastern banks of the Ganges, (4) the establishment of the Raiwala army cantonment on the western banks of the Ganges, and (5) the construction of a National Highway and a railway line, used by an estimated 30,000 motorized vehicles and c. 40 trains per day, respectively (Nandy et al., Reference Nandy, Kushwaha and Mukhopadhyay2007; Rasaily, Reference Rasaily2012).
Methods
Changes in the status of tigers
To assess changes in the status of tigers we relied on triangulating our inferences based on three lines of evidence. (1) We assessed changes in the occupancy of tigers across the landscape by comparing surveys conducted in the winters of 2009–2010 (Harihar & Pandav, Reference Harihar and Pandav2012) and of 2002–2003 (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004) in Tiger Habitat Blocks I and II. (2) We compared an index of tiger sign detections along raus (dry stream beds), based on data from 1995–2010 (S.P. Goyal & A.J.T. Johnsingh, unpubl. data; B. Pandav & A. Harihar, unpubl. data), as they were the only comparable data spanning 15 years. (3) We compiled available estimates of tiger density since 2009.
To assess changes in tiger occupancy in the western Terai Arc Landscape over 7 years, between 2002–2003 (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004) and 2009–2010 (Harihar & Pandav, Reference Harihar and Pandav2012), we compared the data using single-season occupancy models (MacKenzie et al., Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002). In surveys conducted during 2002–2003 (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004), forest ranges were chosen as the basic sampling units, within multiple-use forest divisions and protected areas, and 3–4 sign surveys, with a mean length of 4 km, were conducted along raus. In all, 105 sign surveys, with a total survey effort of 432.5 km, were carried out across 13 administrative units. During 2009–2010, surveys were conducted with greater intensity across the landscape, using more recent analytical approaches (Harihar & Pandav, Reference Harihar and Pandav2012). The survey routes used by Johnsingh et al. (Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004) were included so that comparable datasets could be compiled.
For the purpose of this comparative analysis we considered the forest divisions and protected areas as sampling sites’ and treated the independent sign surveys as sampling occasions’. Although there has been considerable debate about the substitution of spatial subunits for repeated temporal sampling (Kendall & White, Reference Kendall and White2009; Guillera-Arroita, Reference Guillera-Arroita2011), we assumed that each survey route was independent given their spatial configuration and the wide ranging behaviour of tigers. We constructed eight models each for the 2002–2003 and 2009–2010 survey data, taking into account the influence of Tiger Habitat Blocks (B), indices of wild prey (WildP), principal prey (PrincipP; chital Axis axis and sambar Cervus unicolor) and anthropogenic disturbances (Dist) under the single-season occupancy framework. Our objective was to assess the change in occupancy across 7 years and hence we examined differences in the estimates of the occupancy parameter (ψ) derived from the best-supported models.
Before systematic photographic capture–recapture sampling was used to assess the status of tigers in the western section of Rajaji Tiger Reserve, sign surveys along raus were conducted annually. Data for 1995–2005 were obtained from S.P. Goyal and A.J.T. Johnsingh (unpubl. data), and data for 2007–2010 were obtained from Harihar et al. (Reference Harihar, Prasad, Ri, Pandav and Goyal2009) and B. Pandav & A. Harihar (unpubl. data). These surveys were conducted at a rate of 1.25–1.5 km h−1 by teams of 2–4 biologists/trainees and assistants and were 3–4 hours in duration. Each transect was divided into 250 m segments. Indirect evidence of tigers (pug marks, scats and scrapes) was recorded. Based on these data, frequency of occurrence of sign per segment (number of segments with sign/total number of segments surveyed, expressed as a percentage) was calculated per year, and the trend in population was inferred. We also compiled available estimates of tiger density from systematic photographic capture–recapture studies carried out since 2009 (Harihar & Pandav, Reference Harihar and Pandav2012; Rathore, Reference Rathore2015; B. Pandav & A. Harihar, unpubl. data).
Changes in connectivity
The loss of connectivity between Tiger Habitat Blocks I and II is primarily a result of the expansion of Haridwar, Rishikesh and Raiwala townships, and several infrastructural projects. During 1972–1995 an estimated 11.18 km2 of forest in the Chilla–Motichur corridor was lost (Nandy et al., Reference Nandy, Kushwaha and Mukhopadhyay2007). Although tigers disperse from their natal ranges over great distances through a range of forested habitats, agricultural lands and areas of low human population density, they are known to avoid urban areas (Smith, Reference Smith1993; Joshi et al., Reference Joshi, Vaidyanathan, Mondol, Edgaonkar and Ramakrishnan2013; Singh et al., Reference Singh, Qureshi, Sankar, Krausman and Goyal2013). We used remotely sensed data on night-time lighting as an indicator of urbanization (Henderson et al., Reference Henderson, Yeh, Gong, Elvidge and Baugh2003), to evaluate changes in connectivity across the corridor during 1993–2013. We obtained the radiance calibrated data, at a spatial resolution of 30 arc second grid cells (c. 1 km2), for 1993, 2003 and 2013 from the National Geophysical Data Centre (NOAA, 2017). Higher radiance indicates areas of urbanization (i.e. areas through which tigers are less likely to move). The annual mean brightness level in units of 6-bit digital numbers spanning 0–63 were used as resistance values in the analysis. To assess the potential changes in connectivity between the two Tiger Habitat Blocks over time, we used circuit theory (McRae et al., Reference McRae, Dickson, Keitt and Shah2008) implemented in Circuitscape v. 4 (McRae et al., Reference McRae, Shah and Mohapatra2013). We designated the western and eastern parts of Rajaji Tiger Reserve (in Tiger Habitat Blocks I and II, respectively) as nodes in our models, and identified multiple paths of potential connectivity between the nodes using the pair-wise algorithm. We produced cumulative current flow maps for visual representation, and summarized the mean current flow values (a measure of connectivity) for the corridor across the three time periods (1993, 2003 and 2013).
Decision analysis
Clark & Brunner (Reference Clark and Brunner2002) described a decision process that typically comprises seven functions (intelligence, promotion, prescription, invocation, application, appraisal and termination; see definitions in Table 1) that seeks to identify and reconcile any conflicts among policies relating to the implementation of a species recovery programme involving complex partnerships, and minimize the risk of failure. We follow this approach and identify these seven functions in each recommendation given for restoring the Chilla–Motichur corridor, a multi-institutional endeavour, and ascertain the stages at which delay/divergence occurred in the implementation process. Through this analysis we identify the agencies responsible for carrying out the action, with the objective of identifying the functions where intervention was required to strengthen the partnerships and improve the process of recovery.
Table 1 Assessment of decision functions that delayed various conservation actions in the Chilla–Motichur corridor, in India's western Terai Arc Landscape (Fig. 1).

* As provided in Clark & Brunner (Reference Clark and Brunner2002)
Our data are primarily from academic/official publications concerning conservation recommendations for the Chilla–Motichur corridor, covering the period 1986–2016. We initially identified relevant articles from screening the bibliography of Uniyal et al. (Reference Uniyal, Agrawal, Rana and Verma2006), and updated these data by searching ISI Web of Science and Google Scholar. In addition to journal articles and book chapters we searched for non peer-reviewed reports, as many recommendations are communicated through such reports to the relevant implementing agencies. Initially we searched for all articles that included the terms ‘Chilla–Motichur’, ‘Chilla Motichur’ or ‘Chilla–Motichur corridor’ in the title, keywords or abstract. Only articles that focused on the corridor and provided recommendations regarding its restoration were retained for analysis. Furthermore, AH interviewed scientists and bureaucrats engaged with the project, in person or via e-mail, to learn about their experiences and obtain updates about the status of the recommendations. We focus only on those recommendations that have been adopted in government plans.
Results
Decline in tigers
All three lines of evidence confirmed a decline in tiger numbers in Tiger Habitat Block I. The occupancy analysis indicated there had been a 58% reduction in occupancy between the two sampling sessions (Fig. 2a; Supplementary Tables S1–S3). In contrast, in Tiger Habitat Block II there was an increase in occupancy, primarily as a result of the recovery of tigers in eastern Rajaji Tiger Reserve (Fig. 2a; Supplementary Fig. S1; Supplementary Tables S1–S3).
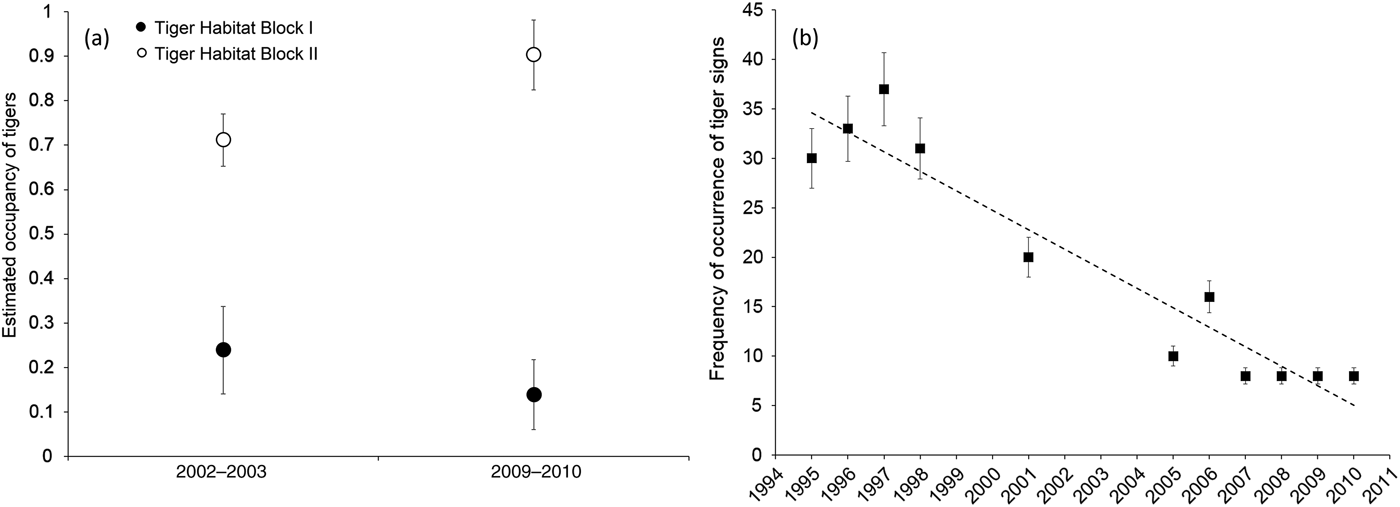
Fig. 2 (a) The estimated occupancy of tigers Panthera tigris in Tiger Habitat Blocks I and II (Fig. 1) during 2002–2003 and 2009–2010, indicating a decline in Tiger Habitat Block I and an increase in Tiger Habitat Block II. (b) Frequency of detection of tiger sign per 250 m segment of transect in the western part of Rajaji National Park during 1995–2010. Error bars indicate 95% CI.
The annual frequency of occurrence of tiger sign per segment indicated a declining trend in the western part of the Reserve (Fig. 2b). During 1995–1998 tiger sign was recorded on c. 30–38% of the surveyed segments. However, during 2007–2010 tiger sign was recorded on only 8% of surveyed segments.
Available estimates of tiger density from systematic photographic capture–recapture sampling conducted across the western part of the Reserve since 2009 indicated the presence of a low-density population of 0.28–0.4 tigers per 100 km2 during 2009–2015 (Supplementary Table S4; Harihar et al., Reference Harihar, Prasad, Ri, Pandav and Goyal2009; Rathore, Reference Rathore2015; Pandav & Harihar, unpubl. data).
Deterioration of habitat in the corridor
Our analysis indicates that since 1993 the opportunity to reconnect tiger subpopulations across the Chilla–Motichur corridor has diminished rapidly (Supplementary Fig. S2). Current flow (a measure of connectivity) across the corridor, estimated through the Circuitscape analysis, decreased progressively and significantly from 1.98 amps (95% CI 1.92–2.03) in 1993 to 1.73 amps (1.67–1.78) in 2003 and 1.25 amps (1.21–1.28) in 2013. The formation of the state of Uttarakhand (in 2000) and the associated economic growth have led to rapid urbanization in the corridor area, and increasing demand on natural resources (Mamgain, Reference Mamgain2007). This habitat deterioration indicates a loss of functional connectivity for tigers.
Decision analysis
We collated 31 articles from 1986–2016 that met our search criteria: 14 peer-reviewed journal articles and 17 non peer-reviewed reports/articles (Supplementary Table S5). Only 20 of these provided specific recommendations to restore connectivity across the Chilla–Motichur corridor, and a review of these indicates a general timeline of recommendations (Fig. 3). Connectivity across the river Ganges between the Chilla (eastern bank) and Motichur (western bank) forest ranges was recognized as being tenuous in the 1980s, particularly with respect to the movement of elephants (Saxena, Reference Saxena1986). In later years conservationists reiterated the need for recovery of this patch so that it could function as a critical corridor for tigers between Tiger Habitat Blocks I and II (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004). Eventually, as the prospect of recovering this patch faded, conservationists subsequently recommended translocation of a breeding male from Tiger Habitat Block II to the western part of the Reserve so that the remaining females could breed (Harihar & Goyal, Reference Harihar and Goyal2010), a recommendation that has yet to be acted upon (Fig. 3). Thus, intelligence has been provided and updated on an ongoing basis to support critical actions required to ensure the persistence of tigers across the two halves of the Reserve.
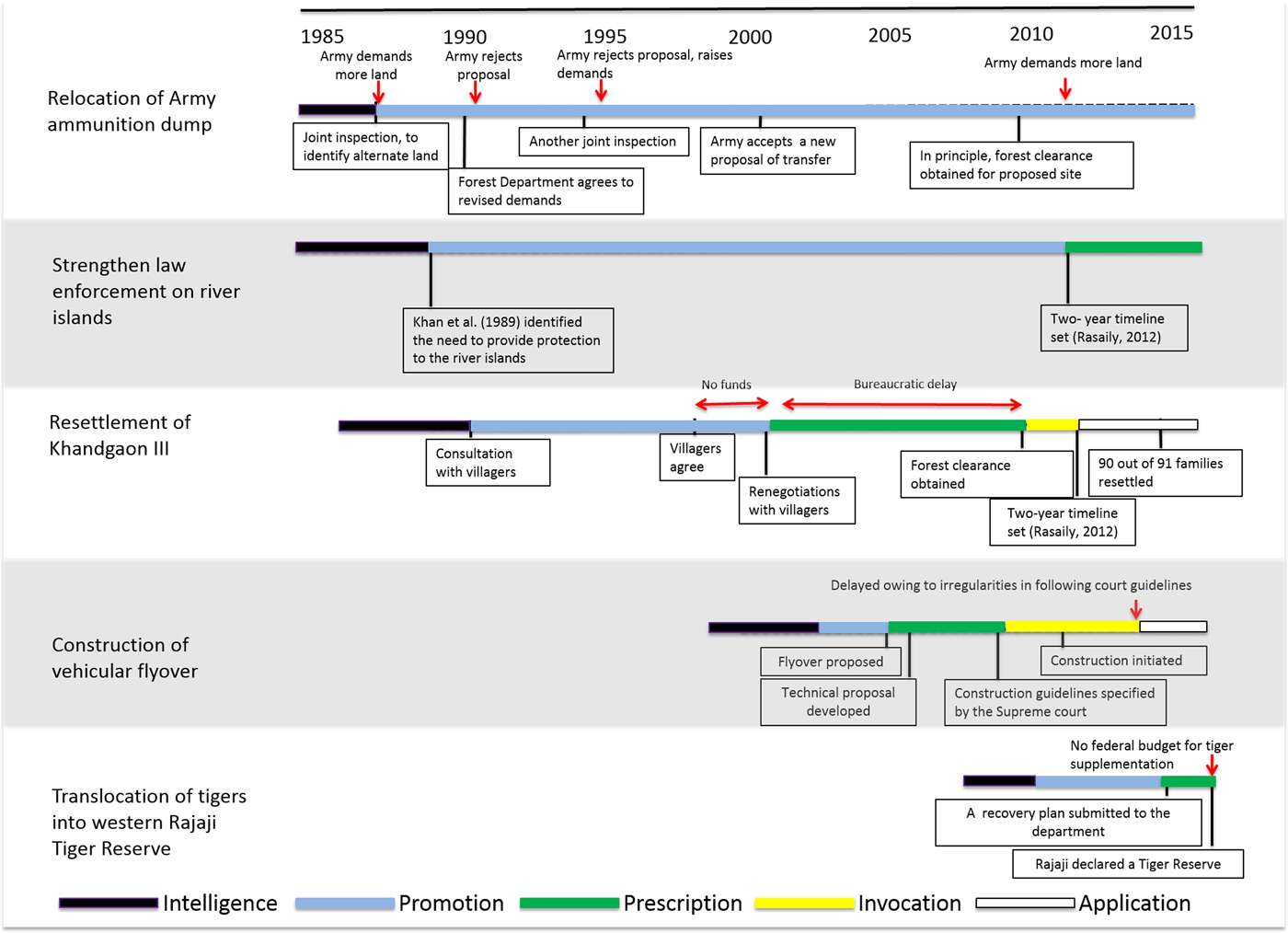
Fig. 3 A timeline for the implementation of key recommendations to restore the Chilla–Mothichur corridor between the western and eastern sectors of Rajaji Tiger Reserve in the western Terai Arc Landscape of India (Fig. 1). Specific actions are outlined within boxes, critical obstacles are denoted by arrows, and the decision functions (following Clark & Brunner, Reference Clark and Brunner2002) are denoted by the colour scheme defined in the legend. For more details regarding the process see Supplementary Material 1.
Overall, the recommendations of conservation scientists provided the broader conservation goals but did not adequately consider the social (e.g. resettlement of Khandgaon III) and political difficulties (relocation of an army ammunition dump) that would be encountered in pursuing these recommendations (Fig. 3). Of the 14 recommendations provided to restore connectivity across the corridor, only five have been promoted and incorporated into government management and operational plans (Rasaily, Reference Rasaily2012; NTCA, 2015), following years of discussion and debate between the partnering agencies to resolve conflicting policies or interests (Fig. 3). Prescribing the recommendations into specific guidelines and actions also required considerable time, as it often necessitated external expertise beyond ecological knowledge of the system. For instance, the recommendation to relocate the ammunition dump needed prescriptions for significant bureaucratic negotiations between the state forest department and Ministry of Defence, and the construction of a flyover was contingent on civil engineering solutions (Fig. 3). For the two recommendations for which implementation was initiated (i.e. resettlement of Khandgaon III and construction of a flyover), the process was delayed by difficulties in mobilizing funds and approvals from concerned state departments, and irregularities in following guidelines for construction, respectively (Fig. 3). In the case of supplementation of tigers, lack of adequate federal funds and political will prevented this prescription from being implemented. Managers are hesitant to implement such a potentially risky action, given that demonstrable population increases post translocation may not happen during their tenure (VanderWerf et al., Reference VanderWerf, Groombridge, Fretz and Swinnerton2006).
Other critical shortcomings included the lack of a clear timeline for each action, and the lack of provision for follow-up actions and evaluation based on a monitoring protocol within an adaptive management framework should difficulties arise in the implementation (Wilhere, Reference Wilhere2002). In the end the only recommendation that was implemented, in 2015, was the resettlement of Khandgaon III village, although the process took 26 years (Fig. 3).
Discussion
The case of the Chilla–Motichur corridor has been described as an acid-test for the Indian conservation movement (Johnsingh et al., Reference Johnsingh, Ramesh, Qureshi, David, Goyal and Rawat2004), and despite the reiteration of recommendations to recover this habitat over nearly 3 decades, the population of tigers at their north-western range limit remains threatened with almost imminent extinction. We found that despite the availability of adequate and timely ecological information and assessment, the project has failed to make progress as a result of multiple institutional failings related to communication and promotion of recommended actions, the lack of responsive governance, ineffective leadership and minimal institutional accountability.
Given that almost all the recommendations required collaboration amongst various organizations with different remits and priorities, the long delays in the initial stages (promotion and prescription) could perhaps have been avoided if a multidisciplinary team had been established at the outset to mobilize resources, identify alternative actions and prescribe the recommendations into specific guidelines and/or actions, with expertise not only in species ecology but in the management of human dimensions in the corridor. Such a multidisciplinary partnership has been instrumental in recovering the eastern barred bandicoot Perameles gunnii (Backhouse et al., Reference Backhouse, Clark, Reading, Clark, Reading and Clarke1994).
Even after prescription, resettlement of Khandgaon III was delayed as the state departments involved struggled to secure adequate funding and collaborate efficiently to implement the identified policies, in a case of diverse mandates meeting layers of official bureaucracy (Fig. 3). Political power play, in which a department of greater strategic interest (e.g. Ministries of Transport and Defence) refuses to make a concession for the requirements of a relatively local issue, was also evident, as is typical of scenarios in which networking and political compromises determine conservation outcomes (Kørnøv & Thissen, Reference Kørnøv and Thissen2000; Lochner et al., Reference Lochner, Weaver, Gelderblom, Peart, Sandwith and Fowkes2003). A key characteristic common to all these delayed actions is that decision making rested within large government agencies with complex and multi-layered bureaucratic structures (Table 2), which made it easy to evade accountability (Wallace et al., Reference Wallace, Clark, Reading and Clark1994; Martin et al., Reference Martin, Nally, Burbidge, Arnall, Garnett and Hayward2012), especially when dealing with long-term conservation goals. Decision makers in government agencies can change frequently; for example, the civil servants who head the management of Rajaji Tiger Reserve change every 5 years.
Table 2 The partners associated with the implementation of each of the recommendations for the conservation of the Chilla–Motichur corridor, in India's western Terai Arc Landscape (Fig. 1), and their specific roles.
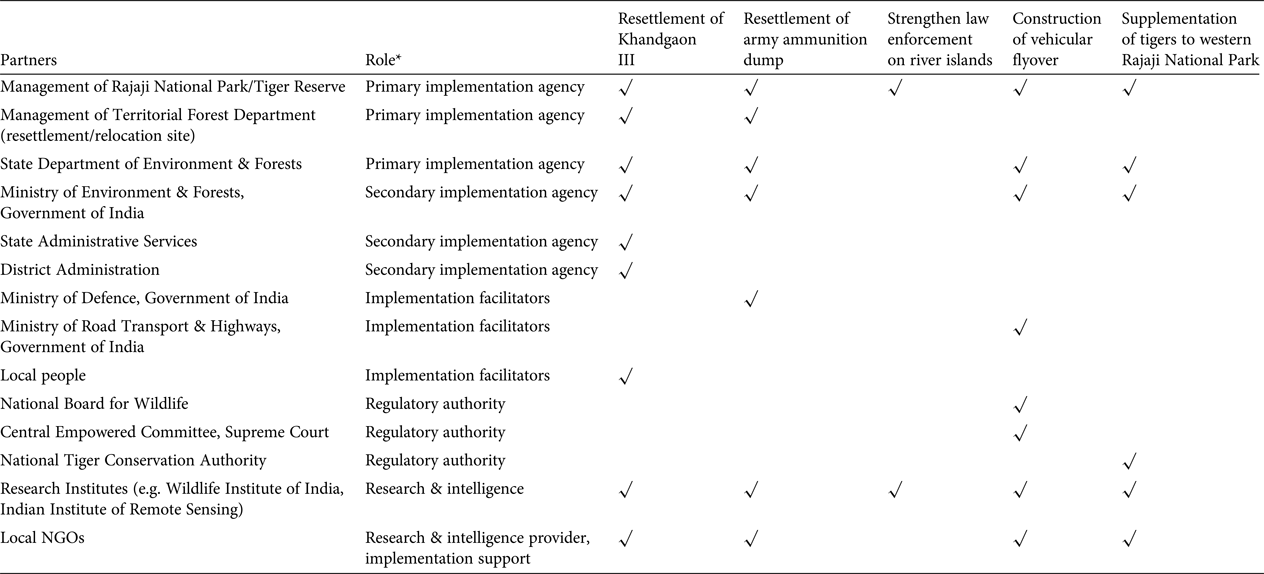
* Primary implementation agency: directly accountable agency with the mandate to conserve wildlife; Secondary implementation agency: facilitate the implementation process through policy and logistic/funding support, but not directly accountable; Implementation facilitators: stakeholders/agencies whose consent and cooperation is indispensable to implementation; Regulatory authority: oversee the process, lay guidelines and resolve any conflicts; Research & intelligence: these NGOs provide the primary evidence and recommendations, and may also provide logistic support for implementation
Effective leadership is of critical importance in recovery efforts for threatened species, to mobilize necessary expert and public participation under one body, garner adequate resources, gain acceptance from relevant government agencies and local communities, champion the cause and take responsibility to effect change in a timely manner (Black & Groombridge, Reference Black and Groombridge2010; Martin et al., Reference Martin, Nally, Burbidge, Arnall, Garnett and Hayward2012). In our case study the primary responsibility for implementing the recommendations rested with multiple agencies within the state forest department, with no clear leadership to ensure that the decision process was implemented in a timely and appropriate manner (Table 2). Moreover, given that many political aspects were clearly not within the programme's sphere of control and were beyond the influence of the primary implementing agencies, effective and flexible leadership was essential to address the constantly changing needs and threats and adapt actions, while focusing on a stable long-term vision to guide the work of the programme (Maris & Béchet, Reference Maris and Béchet2010). Policy czars are widely used in the USA and UK to oversee complex policy reforms that involve multiple government departments, and reach out to multiple stakeholders; these czars cannot be side-lined by self-interested groups as they report to only the highest levels of authority. Such figures may be rare, but conservation could benefit from a better appreciation of leadership. Project designers should bear in mind the need to discuss project objectives and implementation with key stakeholders, and adjust objectives to suit practical and political concerns, perhaps through the adoption of decision-making analysis such as the Delphi approach (MacMillan & Marshall, Reference MacMillan and Marshall2006).
Furthermore, with no adaptive management framework in place, nor any approach to ensure accountability amongst government agencies, this case illustrates the need for conservation programmes to be conceived and managed within a so-called business excellence model (Black & Groombridge, Reference Black and Groombridge2010). This would ensure greater clarity in defining objectives, setting goals, delineating the roles of leaders and staff, identifying success measures and feedback data, creating better links between technical approaches and measures of biological success, community engagement, more effective use of resources, and the establishment of management reviews. In establishing such a system it is critical to develop a good logic model and results chain through the participation of all major stakeholders from the initial stages of any conservation intervention (FOS, 2009; Margoluis et al., Reference Margoluis, Stem, Swaminathan, Brown, Johnson and Placci2013).
The creation and/or restoration of resilient landscape corridors for free-roaming mega-fauna may be an unrealistic goal unless appropriate funding and intervention mechanisms, such as a cost-effective compensation payment scheme or more novel approaches such as certification or payment for ecosystem services schemes, are tied to a bottom-up needs-based development assessment (Harihar et al., Reference Harihar, Veríssimo and MacMillan2015). Only then can such projects appeal to all types of land managers, owners and users, who may not share common objectives, land rights or motivations with either their neighbours or the main project stakeholders (MacMillan & Phillip, Reference MacMillan and Phillip2010; Davies & White, Reference Davies and White2012; Redpath et al., Reference Redpath, Young, Evely, Adams, Sutherland and Whitehouse2013). The entire process of corridor designation could be more efficient if all these factors are synchronized (Brodie et al., Reference Brodie, Giordano, Dickson, Hebblewhite, Bernard and Mohd-Azlan2015).
We have shown how institutional failings caused by uncoordinated policies and actions and perverse decision making, despite awareness of critical ecological knowledge, have conspired to create an imminent extinction crisis for a remnant tiger population in India. The opportunity to restore the Chilla–Motichur corridor seems to be disappearing rapidly, but future conservation projects must pay closer attention to institutional issues that arise from local social, political and economic opportunities and concerns. Simultaneously, they must inherently recognize and address the political idiosyncrasies of the local and national agencies, which are differentially motivated and can easily succumb to self-serving actions, within the decision process.
A key recommendation from our research is the need to develop adaptive conservation plans with effective leadership and funding to secure stakeholder buy-in. The old unicentric model of conservation, in which central decision makers would steer and implement the process, guided by rational use of scientific evidence in a sequential fashion, is no longer appropriate to meet the challenges of a fast-changing and increasingly complex world. The adoption of a polycentric model, in which decisions rest in the hands of multiple independent agencies and outcomes are driven by compromises, available means, political support and power play in an unpredictable fashion, rather than by objective knowledge, may require a cultural shift and a rethink of how conservationists engage with the non-conservation world.
We recommend that greater research effort be invested in understanding decision-making processes in complex conservation projects. Of central importance is the need to recognize that decision making is a process rather than an event, involving a sequence of decisions defined by rules, which seek to reconcile policy differences between multiple stakeholders with differing and conflicting motivations. We believe that a firmer understanding of decision-making science, and investment in this discipline, could generate considerable benefits for biodiversity.
Postscript
In June 2017 the National Tiger Conservation Authority allocated a budget of INR 34 million (USD 500,000) for translocating tigers into Rajaji Tiger Reserve. It is now expected that five tigers will be translocated in the winter of 2017–2018 to supplement the existing population and assist its recovery (Thapliyal, Reference Thapliyal2017).
Acknowledgements
We thank A.J.T Johnsingh, S.P. Goyal and Bivash Pandav for helping us at various stages in the preparation of this manuscript. This study was supported in part by grants from Kaplan Graduate Awards (Panthera), Kathryn Fuller Science for Nature Fellowships (WWF) and the Rufford Small Grants Foundation (Grant 10691-2) to AH.
Author contributions
AH, MG-H and DCM conceived the study and wrote and edited the article. AH and MG-H collected and analysed the data.
Biographical sketches
Abishek Harihar has worked on tiger conservation in northern India since 2003 and currently works as a tiger population ecologist, with research interests spanning population ecology, law enforcement monitoring, measuring conservation effectiveness, and conservation decision making. Mousumi Ghosh-Harihar has previously researched the ecological and historical determinants of breeding leaf warblers in the Himalayas. She has also worked on urban birds, human–tiger conflict, and formulating an online photographic data-sharing policy. Douglas MacMillan’s work focuses on understanding, modelling and evaluating the economic dimensions of environmental change and biodiversity conservation, with special expertise in ecosystem valuation, decision analysis and conflict between people and large carnivores.







