Introduction
Mikania Mikania micrantha is a fast-growing perennial climber, capable of producing large amounts of biomass, and is highly invasive in humid tropical and subtropical regions of Asia and the Pacific (Waterhouse, Reference Waterhouse1994). It is commonly called mile-a-minute weed because of its exceptionally fast growth rate (Holm et al., Reference Holm, Plucknett, Pancho and Herberger1977). It is a major threat to agriculture and biodiversity as it is reported to smother and retard or kill a wide variety of young trees, crops, native plants and fodder grasses (Palit, Reference Palit1981; Muniappan & Viraktamath, Reference Muniappan and Viraktamath1993; Muraleedharan & Anitha, Reference Muraleedharan and Anitha2000). A native to tropical and subtropical Central and South America, mikania usually only occurs in low abundance in these regions (Barreto & Evans, Reference Barreto and Evans1995).
Mikania was first reported in the east of Nepal in the early 1960s (Tiwari et al., Reference Tiwari, Adhikari, Siwakoti and Subedi2005) and appears to be spreading aggressively westwards along the terai (grassland–forest) habitat zone in the south of Nepal (Poudel et al., Reference Poudel, Baral, Ellison, Subedi, Thomas and Murphy2005) where it has become a major threat to protected areas. Anecdotal evidence suggests it may have spread from Assam, India, to east Nepal through the transport of tea seedlings (Tiwari et al., Reference Tiwari, Adhikari, Siwakoti and Subedi2005). It probably reached Chitwan National Park in the early 1990s as it was identified there in 1997 at a low density and seems not to have been seen by forest guides a few years prior to this (H. Evans, pers. comm.). It is now the principal invasive plant in the Park and is widespread and abundant particularly in areas closer to the main rivers, the Rapti flowing east to west and the Narayani flowing into the Rapti from the north-east. Qualitative observations by the National Trust for Nature Conservation in recent years suggest that the Indian rhinoceros Rhinoceros unicornis, also known as the greater one-horned rhinoceros, and other large herbivore populations have declined in areas with high mikania infestation. Thus mikania is a major concern as it has the potential to destroy prime habitats of threatened and important species in Chitwan National Park, a UNESCO World Heritage Site.
Mikania is a fast-growing climber with a high reproductive rate (sexual and asexual) and is also fire-adapted. Asexual reproduction is from roots that develop from nodes on small sections of the stem. Whereas these natural biological characteristics give the plant the potential to spread, anthropogenic factors can either cause or greatly exacerbate the actual spread and growth of mikania; this is in common with some other invasive plant species in the Indian subcontinent (Murphy, Reference Murphy, Sankaran, Murphy and Evans2001). In particular, studies have shown that burning of vegetation and manual weed control for crops, common practices in the lowland grasslands of Nepal and India, can promote spread. For example, in shifting agriculture in north-east India, after slash and burn in forests, mikania vigour was greater than in unburnt plots, indicating that mikania can survive after fire (Swamy & Ramakrishnan, Reference Swamy and Ramakrishnan1987a, Reference Swamy and Ramakrishnan1988). In addition, it has been shown that mikania is more efficient than most native plants at utilizing important nutrients when these and light are not limiting (Swamy & Ramakrishnan, Reference Swamy and Ramakrishnan1987b), and after burning nutrients are often plentiful. Moreover, efforts at manual cutting and removal can often promote local dispersal from regeneration of small plant sections that are dropped, as has been reported from south China (Wang et al., Reference Wang, Li, Zhang, Zan, Zhang, Liao, Zhang, Zhou, Pang, Zhang, Gu and Pang2003) and India (Kerala Forest Research Institute, pers. comm.).
In view of the threat of mikania to species of conservation concern in Chitwan National Park a draft management plan for the plant is under development by the Department of National Parks and Wildlife Conservation and National Trust for Nature Conservation, with support from CAB International and the Zoological Society of London. Herbicides cannot be used in the core of the Park because of the risk of side effects (DNPWC, 2009) and thus a different approach is needed. As a basis for a management plan, studies on mikania ecology and dispersal have been conducted to identify factors that could be driving or exacerbating the invasion, and a special emphasis has been placed on anthropogenic factors, as the core area of the Park is used by local people for resource extraction. This information will be used to prioritize areas for immediate control of mikania and to design an initial framework of interventions based on cultural and mechanical controls that involve local communities. Such studies will be of direct relevance to other affected protected areas. Here we report on the current extent of the mikania invasion in Chitwan National Park, particularly in vegetation zones important for the Indian rhinoceros, and on the activities of communities in the buffer zone that may exacerbate mikania infestations.
Study area
Chitwan National Park (Fig. 1) was established in 1973 as the first national park in Nepal and is situated in a valley bounded by the Siwalik Hills in the terai region (Sapkota, Reference Sapkota2007). The Park includes a core conservation area of 932 km2 and an additional 750 km2, surrounding the core area, set up as a buffer zone. Climate is subtropical monsoonal, with a mean annual rainfall of 2,100 mm. The Park supports a wide variety of plants, fish, reptiles, birds and mammals. The habitats can be broadly classed into: Sal Shorea robusta forest, riverine/subtropical mixed hardwood forests (e.g. Trewia nudiflora, Bombax ceiba and Dalbergia sissoo), tall grasslands (e.g. Saccharum spontaneum, Narenga porphyracorma, Phragmites karka), short grasslands (e.g. Imperata cylindrica), wetlands (including lakes) and shrublands (Laurie, Reference Laurie1982; Dinerstein & Price, Reference Dinerstein and Price1991; DNPWC, 2009). The riverine/subtropical mixed hardwood forests and grassland habitats tend to form a mosaic in the moister areas of the Park.

Fig. 1 Chitwan National Park, main rivers and surrounding buffer zones, and the locations of the sample of five Village Development Communities (VDCs; Table 1). The rectangle on the inset indicates the location of the main map in southern Nepal.
The buffer zone was delineated in 1996 with the aim of creating local ownership and involving communities in conservation. The buffer zone communities receive part of the total income of the Park for local development and conservation (DNPWC, 2006a). The buffer zone includes 37 Village Development Communities of Chitwan and Nawalparasi districts around the Park, with > 250,000 inhabitants. Ethnically, the communities consist mostly of hill migrants and indigenous people, the latter include Tharu, Bote and Musahar. The buffer zone communities are allowed to enter the core area of the Park for a few weeks early in the year to collect resources such as fodder grasses. They also burn parts of the core area to encourage the regeneration of grasses.
Methods
A national rhinoceros census conducted in May 2008 (DNPWC, 2009) provided an opportunity to obtain information on the level of invasion of mikania while saving time and resources. In summary, the rhinoceros census in Chitwan National Park was conducted by dividing all potential rhinoceros habitats into 16 contiguous blocks situated east to west in the northern and central part of the Park, as the habitat in the south is generally too dry and hilly for rhinoceroses. These blocks were surveyed by observers on elephants. There was one observer per elephant and the elephants moved in parallel through the blocks along transects, the block size determining the number of elephants used. The distance between two elephants was maintained at c. 100–200 m in open grasslands and 50 m in dense forests, to ensure areas were thoroughly covered. The census covered most of the core area of the Park, buffer zone community forests and the Barandabar Forest corridor (in the north-eastern boundary of the Park) as this is also an important conservation area.
The method for measuring mikania infestation involved the assessment of mikania cover by each observer within an approximately semi-circular plot of 50 m in front, left and right of the elephant (except in dense forest where every other observer made the observations).The level of infestation was measured using a simple ranking of cover within the area as: 0, absence; 1 ⩽ 50%; 2 > 50%. Assessments were made every c. 30 minutes during the census and thus sampling was approximately proportional to the area covered by each habitat.
The type of habitat and position of each plot were recorded using a geographical positioning system. The habitats that rhinoceroses are usually found in were divided into five types: riverine/subtropical mixed hardwood forests, Sal forest, tall grassland, short grassland, and wetland (DNPWC, 2009). Over 40 observers were trained to assess the level of mikania invasion and record the habitats in which the plant occurred. The information on the recorded sheets was checked and entered into a spreadsheet at the end of each survey day. The level of invasion of mikania was summarized as frequencies of plots invaded in each habitat. Rhinoceros count data was similarly summarized in relation to habitats assessed. The mikania data was mapped using ArcGIS v. 9.3 and 10 (ESRI, Redlands, USA).
A broad assessment of the possible role of anthropogenic factors in the spread of the plant was made during a general survey of buffer zone communities adjoining the northern part of Park, who use the Park for resource collection (NTNC, 2009). Rai et al. (Reference Rai, Scarborough, Subedi and Lamichhane2012) describe methods used for the general survey but specific methods relevant to the assessment reported here, conducted on the same communities, are as follows. Two main groups of questions were posed: (1) what resources are collected by communities that could serve as vectors for invasive plant material from the core area of the Park and how far do people travel into this area, and (2) do communities burn vegetation in the core area of the Park and if so, why, by how much, and when?
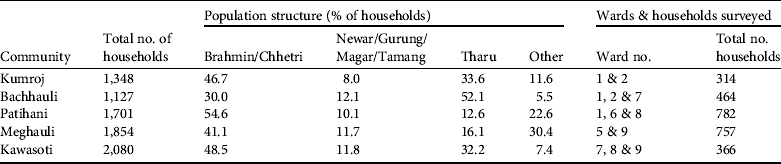
Five Village Development Communities were selected for the community survey. Selection was based on two main criteria: being adjacent to the Park and having a high proportion of indigenous/ethnic people (e.g. Tharu, Bote, Darai). The last point is based on the assumption that ethnic communities are more dependent on natural resources for their livelihood compared to other communities. Using these criteria, the Village Development Communities of Kumroj, Bachhauli, Patihani and Meghauli from Chitwan district and Kawasoti from Nawalparasi district were selected for the survey (Fig. 1). The ethnic composition of each is shown in Table 1. From each Communitiy two to three wards adjacent to the Park were selected. A total of 2,683 households were surveyed across the five Communities (Table 1).
Table 1 Ethnic composition of the sample of five Village Development Communities (Fig. 1) and the wards surveyed in each Community.
Information about the questions was collected using a structured questionnaire. From each Village Development Community c. 30 respondents (one respondent per household) were interviewed. Households were selected from numbered voting lists of the wards; a household was selected randomly from each ward and then every 10th household selected after this.
The survey was conducted from April to May 2009 and the questionnaires were completed through direct interviews with the respondents. Two researchers were involved in the questionnaire survey, one interviewer and one recorder. Summaries of respondents' answers were generated with SPSS v. 11.5 (SPSS Inc., Chicago, USA).
Results
Assessment of mikania distribution and abundance
The assessment of mikania distribution and level of invasion was undertaken in a total of 1,565 locations. The area surveyed was 470 km2 and took 3,107 elephant hours to complete. As the assessments were taken uniformly across the habitats, the total number of assessed plots in each habitat provides a relative measure of the geographical size of the habitats (Table 2). Overall, 44% of plots contained mikania and a high invasion rate (> 50%) was recorded in c. 15% of the plots (Table 2). The distribution of levels of invasion of mikania across Chitwan National Park is shown in Fig 2.

Fig. 2 The distribution of infestation levels of mikania Mikania micrantha in Chitwan National Park (NP) and surrounding buffer zones, and the locations of the sample of five Village Development Communities (VDCs; Table 1).
Table 2 Percentage invasion of main Indian rhinoceros Rhinoceros unicornis habitat types by mikania Mikania micrantha in Chitwan National Park (Fig. 1), based on assessed plots.
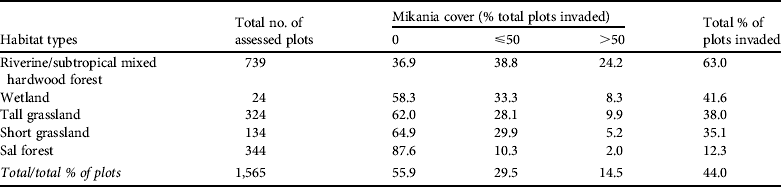
Riverine/subtropical mixed hardwood forests were found to be most invaded by mikania (63.1% of plots invaded, 24.2% of the plots highly invaded). Habitat types in descending order of magnitude of invasion were wetland, tall grassland, short grassland and Sal forest (Table 2).
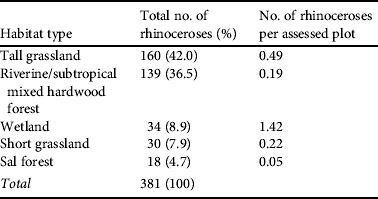
The locations of 381 rhinoceroses recorded across the five habitats are shown in Table 3. In terms of numbers of individuals, 87% of the rhinoceroses recorded were sighted in tall grasslands, riverine/subtropical mixed hardwood forests and wetlands. These habitats also have some of the highest levels of mikania invasion (38, 63 and 42% of the plots in these habitats; Table 2). Rhinoceros densities in relation to the various habitat types were also examined, using an index estimated from the number of assessed plots per habitat (Table 2), as each habitat type occupied a different percentage of the sampling area. This analysis (last column, Table 3) indicates the importance of wetlands for the rhinoceroses and these habitats are the second-most highly invaded by mikania.
Table 3 Distribution of rhinoceroses by habitat type in Chitwan National Park, as recorded in the May 2008 national rhinoceros census. The last column gives an index of rhinoceros density per habitat estimated from the number of assessed plots per habitat (Table 2).
Human activities and mikania spread
A total of 156 respondents, 53% male and 47% female, were interviewed, which represented c. 6% of the total number of households in the wards surveyed (Table 1). The highest numbers of respondents were from Tharu, Bote and Musahar communities (45%) followed by Brahmin and Chhetri communities (32%), Magar, Gurung and Tamang communities (17%), Newar and other communities (6%). The primary occupation of > 85% of the respondents was farming. The survey included community leaders and elderly people and it was found that they generally had the most in-depth knowledge about the questions being asked.
The survey results showed that people living in the buffer zone depend on the core area of the Park for resources such as fuelwood, fodder, thatching materials, vegetables, fish and grazing. The level of dependency on each of these resources varies across the Village Development Communities and settlements but in most cases dependency on the core area of the Park was high compared with sources from community and private forests. An example is fodder (which is highly infested with mikania) collection, which all Communities (except Kawasoti) were highly dependent on in the core area (Fig. 3). The mean amount (kg) of grass removed from the core area per household per annum by the Village Development Communities (except Kawasoti) is shown in Fig. 4. From the interviews it was clear that in general local people do not remove mikania from collected fodder grass before transport.

Fig. 3 The number of respondents in each Village Development Community who collect fodder from the core area of Chitwan National Park (Fig. 1) compared to those who collect fodder from other areas (NTNC, 2009).
The movement of local people inside the core area of the Park varied with season and the availability of targeted resources but it was clear that the period during which people entered the core area was much greater than that permitted by the authorities. Some of the distances travelled by local people can be large; e.g. the people of Bachhauli Village Development Community travel an average of 8.4 km and people of Patihani travel an average 5.9 km for thatch grasses. In general, people across the Village Development Communities (not including Kawasoti) travel the furthest for thatch grasses and shortest distance for fuelwood (Fig. 5).

Fig. 5 Mean ± SD distance (km) travelled by people to collect fuelwood, fodder, thatching and wild vegetable resources in the core area of Chitwan National Park (Fig. 1) (NTNC, 2009).
Burning is a traditional practice in the core area of the Park. The reasons given by the respondents for burning grasslands were, in descending order, to expose cane for collection, generate new grass shoots for elephants and for thatch, honey gathering, and accidental burning. Approximately 55% of respondents claimed that burning generally takes place during February–April but c. 35% claimed April–May (Fig. 6). In practice most of the areas are burnt during March–April (NTNC, 2009). The late fires during the last part of April and May are hazardous because of the hot and dry conditions.
In four of the five Village Development Communities most respondents reported that 25–50% of the core area is burnt (Fig. 7); in the Bachhauli Village Development Community most respondents reported that 50–75% of the core area is burnt. The extent of burning largely depends on the season, the amount of pre-monsoon rainfall and the efforts of Park authorities to control the fire. During the dry season (March–May) of 2009 > 75% of the Park was burnt severely as it was one of the driest years of the decade (NTNC, 2009). Only wetlands and moist riverine/sub-tropical hardwood forests remained unburnt, because of their high moisture.
All of the respondents agreed that burning in and around the Park has been taking place for > 20 years. Forty-five percent of the respondents reported that burning has either decreased or not changed compared to the past but only 11% of the respondents claimed that burning has increased.
Discussion
Chitwan National Park contains habitats vitally important for the conservation of the Indian rhinoceros and many other species characteristic of the terai region. Of crucial importance is the unique grass–tree–wetland mosaic on which the rhinoceros depends. An earlier study by Sapkota (Reference Sapkota2007) has demonstrated the negative impact that mikania has on plant species. For example, in highly-invaded grassland many I. cylindrica and S. spontaneum plants were found dead and no new culms were observed sprouting from the rootstocks, and in highly invaded riverine/subtropical hardwood forests saplings of B. ceiba, D. sissoo and Acacia catechu had died and no regeneration was observed.
Although rhinoceroses forage and roam across a number of habitat types in the Park (Laurie, Reference Laurie1982) they are most frequently found in the mosaics of tall grassland and riverine/subtropical mixed hardwood forest (Laurie, Reference Laurie1982; Dinerstein & Price, Reference Dinerstein and Price1991). Similar observations have been made in Bardia National Park (Steinheim et al., Reference Steinheim, Wegge, Fjellstad, Jnawali and Weladji2005). A key component of the tall grasslands are Saccharum spp., especially S. spontaneum, and these form a major part of the rhinoceros’ diet (Laurie, Reference Laurie1982; Steinheim et al., Reference Steinheim, Wegge, Fjellstad, Jnawali and Weladji2005) but rhinoceroses also browse shrubs and the leaves, twigs and fruits of trees and saplings.
Forty-four percent of the rhinoceros habitats in Chitwan National Park are now affected by mikania. The riverine/subtropical mixed hardwood forests, tall grass and wetland habitats are the most highly invaded and this is where the highest number (87%; Table 3) of rhinoceroses were recorded. No quantitative estimates could be made of the extent to which mikania may already be affecting rhinoceros numbers in these habitats but anecdotal evidence does exist on the impact of mikania on the rhinoceros populations. Data from regular monitoring in the Baghmara and Chitrasen buffer zone community forests suggest a negative impact. In these areas > 30 rhinos used to be seen regularly in the late 1990s but now < 15 rhinoceroses are known to utilize them. The decline does not appear to be because of poaching as there have been extensive surveys in the area and only a few carcasses have been detected (NTNC, unpubl. data).
Rai et al. (Reference Rai, Scarborough, Subedi and Lamichhane2012) report that in all the buffer zone communities studied people seemed to be aware of and recognize mikania; there was also a broad consensus that mikania is likely to be having a severe negative effect on natural resources. Overall, the communities reported that mikania is spreading rapidly. It was also clear that mikania is not utilized in any significant way apart from supplementing fodder grasses for goats during the dry period. Twenty-three percent of the households sampled indicated that mikania is collected for fodder on an ad hoc basis from the core Park and community forests (NTNC, 2009; Rai et al., Reference Rai, Scarborough, Subedi and Lamichhane2012).
In our socio-economic study all the Village Development Communities (apart from Kawasoti) had a high dependency on the Park for livestock fodder, thatching material, fuelwood, vegetables and grazing. Livestock are an integral part of traditional subsistence farming and most of the households own livestock. Mikania is common across much of the grassland areas and is not necessarily removed from the grass cut for fodder.
People enter the core area of the Park to collect resources during a significant period of the year, whereas the permitted entry period is only a few weeks in the early part of the year (NTNC, pers. comm.). The distances travelled by people for resources varied between the Village Development Communities and the resource being collected but mean distance for each resource is at least 4 km. It is known that mikania can be spread accidentally during cutting (Wang et al., Reference Wang, Li, Zhang, Zan, Zhang, Liao, Zhang, Zhou, Pang, Zhang, Gu and Pang2003) and thus the risk of inadvertent spread throughout the Park in the course of community activities is high.
Of more significance as a factor generating the growth and spread of mikania is probably the burning of vegetation by those who live and work in and around the Park. The majority of people interviewed reported that 25–50% of the core area of the Park is burnt annually. Burning is uncontrolled and new mikania growth on burnt patches is frequently seen after the main monsoon, probably having sprouted from unburnt root systems but also from wind-blown seed. This new growth will have an advantage over native plant species because of the plentiful nutrient supply and good sunlight.
Our survey confirms that mikania is established in a wide area in the core of the Park, in places totally smothering native vegetation, and thus the potential for mikania to destroy prime habitat for rhinoceroses is high. This study also indicates that human activity is exacerbating mikania spread and growth through collection and transport of the plant and through the annual burning of the Park. These factors have important and direct implications for a management plan for controlling mikania in the core of the Park. There is a clear need to increase awareness among communities and policy makers about the impacts of mikania on natural resources and the risk of inadvertent spread of the plant if cut material is moved around. But the main issue that needs to be addressed is the extensive and uncontrolled burning that takes place each year. Ideally, the burning needs to be controlled such that native vegetation in the Park has a chance to survive and out-compete mikania, which is fire-adapted. One option would be to restrict burning of some areas important for the rhinoceros to once every few years; some controlled burning is important to maintain the grassland habitats in the longer term but further research is needed to determine the optimal regime for burning. Protection of areas from burning can be achieved by the use of fire breaks but the local communities and others who set fires need to be engaged in the plan, to avoid accidental burning of these areas. The spread of mikania westwards also needs to be monitored; this can be done by the establishment of permanent sampling plots throughout the Park. It will be important to include the general management measures recommended here in relevant conservation action plans, such as that for the rhinoceros (DNPWC, 2006b).
Furthermore, a greater understanding of the impacts and dynamics of mikania in relation to major habitats is required. The extent of invasion in different plant communities appears to vary, with some grassland types badly affected. This information is necessary to justify longer-term intervention options such as biological control, as this is the most viable and sustained solution and will complement shorter-term measures. The most promising biological control agents are three rust species from the neotropical native range of the weed (Ellison, Reference Ellison, Sankaran, Murphy and Evans2001), which kill the leaf and the stem, affecting the whole plant. The highly selective rust fungus Puccinia spegazzinii, which can only survive on mikania, is being introduced into India and China and lessons drawn from these countries will be invaluable (Ellison & Murphy, Reference Ellison and Murphy2001; Sankaran et al., Reference Sankaran, Muraleedharan and Anitha2001). The threat of invasive alien species to biodiversity is a problem that may undermine other conservation efforts. Actions must be put in place to address this issue but these must be based on a sound understanding of the biology of invasions.
Acknowledgements
The authors would like to thank the UK government's Darwin Initiative and WWF, Nepal. Retired DNPWC Director General Mr Bajimaya and all the census participants are sincerely thanked for their continued commitment to conservation of the Indian rhinoceros.
Biographical sketches
Sean T. Murphy studies the biology of populations, factors generating outbreaks, and the development of prevention and management methods. Naresh Subedi runs biodiversity research and monitoring projects in Nepal. Shant Raj Jnawali specializes in rhinoceros, tiger and elephant population management in human-dominated landscapes in Nepal. Babu Ram Lamichhane is interested in habitat dynamics and field implementation of community engagement projects. Gopal Prasad Upadhyay works on management of sustainable protected areas through people's participation. Richard Kock focuses on wildlife health and emerging diseases. Raj Amin specializes in Asian and African grassland and forest ecosystems and the development of long-term conservation projects.















