Introduction
Amphibians are the most threatened group of vertebrates, with multiple causes implicated in their decline, including the emergence of new pathogens (Blaustein et al., Reference Blaustein, Gervasi, Johnson, Hoverman, Belden, Bradley and Xie2012), biological invasions (Kats & Ferrer, Reference Kats and Ferrer2003), climate change (Carey & Alexander, Reference Carey and Alexander2003) and habitat loss and alteration (e.g. as a result of intensive vehicle traffic; Cushman, Reference Cushman2006). At the individual level the consequences of habitat alteration are potentially multiple and may include a range of morphological (Todd & Rothermel, Reference Todd and Rothermel2006) and physiological responses (Narayan, Reference Narayan2013).
Reduced body size (a trend toward dwarfism) as a result of habitat alteration has been reported (e.g. Todd & Rothermel, Reference Todd and Rothermel2006), and may be a result of maternal effects (e.g. decrease in energy investment in breeding), ontogenic factors during the larval phase (e.g. changes in larval density or thermal condition) or life history traits (e.g. decreased survival after metamorphosis affecting age structure and size pattern). Furthermore, studies have shown that a decline in body condition may be caused by modifications of habitat structure and trophic productivity (Denoël & Poncin, Reference Denoël and Poncin2001; Janin et al., Reference Janin, Léna and Joly2011). These changes in body size and condition may directly or indirectly cause a decrease in fitness (Altwegg & Reyer, Reference Altwegg and Reyer2003).
Few studies have provided evidence that habitat alteration influences endocrine stress responses in amphibians (for a review see Narayan, Reference Narayan2013). Maintaining homeostasis (i.e. the stability of physiological systems that maintain life) through environmental changes depends on a set of mechanisms called allostasis (maintaining stability through change; McEwen & Wingfield, Reference McEwen and Wingfield2003). In vertebrates the main allostasis mediators are glucocorticoid hormones (e.g. corticosterone), which are usually considered an indicator of endocrine stress response (Moore & Jessop, Reference Moore and Jessop2003). These hormones play a critical role in blood glucose regulation but also perform a range of other functions, including effects on intermediary metabolism, immune function, behaviour, electrolyte balance, growth and reproduction (Hill et al., Reference Hill, Wyse and Anderson2008).
Analysing and understanding how habitat alteration may shape morphological and physiological responses in amphibians is an important challenge for conservation strategies. We examined the influence of intensive vehicle traffic (motorbikes and trucks on unpaved pathways) on the body size and condition and the production of glucocorticoids (i.e. corticosterone) in the yellow-bellied toad Bombina variegata. Although this species is categorized as Least Concern on the IUCN Red List (Kuzmin et al., Reference Kuzmin, Denoël, Anthony, Andreone, Schmidt and Ogrodowczyk2009; IUCN, 2015), populations have experienced significant decline in Western Europe during the 20th century (Lescure et al., Reference Lescure, Pichenot and Cochard2011). Several factors, such as habitat loss and changes in farming practices, have been proposed to explain this decline (Canessa et al., Reference Canessa, Oneto, Ottonello, Arillo and Salvidio2013). For nearly a century the increasing mechanization of forestry, agricultural and mining activities has contributed to landscape change and habitat availability. In particular, these activities create water bodies (e.g. ruts, residual puddles on unpaved pathways) in which B. variegata can reproduce (e.g. Cayuela et al., Reference Cayuela, Besnard, Bonnaire, Perret, Rivoalen, Miaud and Joly2014). These semi-natural environments are mainly characterized by a low water volume with a high risk of drying up (Kopecky et al., Reference Kopecky, Vojar and Denoël2010), and few competitors and predators (Barandun & Reyer, Reference Barandun and Reyer1998), and therefore they fulfil the biotic and abiotic conditions that prevail in natural water bodies used for breeding by B. variegata (Cayuela et al., Reference Cayuela, Cheylan and Joly2011, Reference Cayuela, Besnard and Joly2013). Furthermore, they may compensate locally for the loss of natural habitats as a result of agricultural development and urbanization (Cayuela et al., Reference Cayuela, Lambrey, Vacher and Miaud2015). However, the benefits provided by the availability of these substitute breeding sites could be balanced by costs such as intensive vehicle traffic on unpaved pathways, which may damage breeding and terrestrial habitats. Using data collected from four populations we tested the hypothesis that intensive vehicle traffic (motorbikes and heavy trucks) negatively influences both body size and body condition among yellow-bellied toads. We also postulated that such traffic could lead to an increase in corticosterone production.
Methods
Field sampling
The study was conducted in May 2014 in four populations (corresponding to distinct clusters of water bodies) in Savoie and Haute-Savoie, France (Fig. 1). We selected two populations that were exposed to intensive vehicle traffic (all-terrain vehicles, including motorcycles and heavy trucks) at least once per day, and two control populations, which were not exposed to traffic. We assumed that the four populations were comparable, considering latitude and altitude (Table 1). In exposed populations (POP1 and POP2) the toads bred in ruts and shallow ponds in two stone quarries, which were continuously damaged by all-terrain vehicles and trucks. In control populations (POP3 and POP4) breeding took place in ruts and shallow ponds in a wet meadow and in a stone quarry that had not been exploited since 1993; access for vehicles to these sites was limited. The four populations were sampled over a 2-day period; one exposed and one control population were sampled per day. Only sexually mature individuals were captured, and sex was identified on the basis of stronger forearms and the presence of nuptial pads in males. Adults were captured at random in the daytime, by hand or using a dip net (Table 2). To measure corticosterone production, within 3 minutes of capture a dry cotton ball of known weight was inserted into the mouth for 30 s to collect saliva. As changes in corticosterone concentration as a result of capture are detectable after 30 minutes (Ricciardella et al., Reference Ricciardella, Bliley, Feth and Woodley2010), we assumed that baseline corticosterone measurements were not biased by manipulation in the field. The toads were then measured, weighed using a precision electronic balance, and released. Sample size per sex and population, and descriptive statistics of body size (snout–vent length), weight and baseline corticosterone concentrations are presented in Table 2.

Fig. 1 Locations of two populations of yellow-bellied toads Bombina variegata exposed to intensive vehicle traffic, and two control populations, in Savoie and Haute-Savoie, France.
Table 1 Details of two populations of yellow-bellied toads Bombina variegata exposed to intensive vehicle traffic, and two control populations, in Savoie and Haute-Savoie, France, with population status, vehicle type, altitude and habitat type.
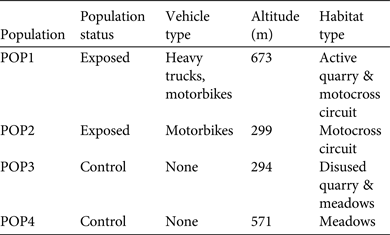
Table 2 Sample sizes (n) of male and female yellow-bellied toads in two populations exposed to intensive vehicle traffic (POP1 and POP2) and two control populations (POP3 and POP4), with descriptive statistics for body size (snout–vent length), mass and baseline corticosterone concentrations in saliva.
Dosing corticosterone levels
Corticosterone in saliva samples was analysed by enzyme-linked immunoassay, using protocols validated previously for amphibians (Janin et al., Reference Janin, Léna, Deblois and Joly2012). Each cotton ball was weighed, put in a microtube equipped with a filter to retain the cotton fibre during centrifugation, and stored at −80°C. Samples were reconstituted by addition of 150 μL of phosphate buffer (1 M phosphate solution containing 1% bovine serum albumin, 4 M sodium chloride, 10 mM ethylenediaminetetraacetic acid and 0.1% sodium azide) and centrifuged. Samples were diluted and analysed in duplicate using a 96-well colometric enzyme-linked immunoassay kit (500651, Cayman Chemical Company, Ann Arbor, USA). Coloration was evaluated using a spectrometer (Absorbance Microplate Reader ELx808, Biotek, Winooski, USA) at 405 nm wavelength The concentration of corticosterone in saliva samples was calculated using a standard curve run on each plate. We estimated the corticosterone concentration in 1 mg of saliva by dividing the measure in samples by the weight of saliva collected.
Statistical analysis
Prior to analysis all continuous variables (i.e. body size, weight and corticosterone concentration; Table 2) were zero-centred to fit a normal distribution. Body size was log-transformed to model body condition. We investigated whether body size (snout–vent length, SVL) and corticosterone level (CORT) varied between the sexes (SEX) and according to exposure to traffic (STATUS: exposure to traffic vs control situation). For that purpose we used linear mixed models, treating SVL and CORT as the dependent variables. The nominal variables SEX and STATUS and their interactive effect were introduced as explanatory terms in the fixed part of the model, and the population of origin was introduced as a random effect. To examine the influence of STATUS on body condition according to sex we performed a covariance analysis using body weight (WEIGHT) as the dependent variable and SVL as the adjustment covariate. SEX, STATUS, SVL and all their interactive effects were thus introduced as explanatory terms in the fixed part of the model, and the population of origin was introduced as a random effect. All analyses were performed using restricted maximum likelihood optimization. Firstly, we checked variance homogeneity between the two population statuses. For that purpose we specified a distinct error term for each status in the covariance structure of the mixed model and we used a likelihood ratio test to estimate the significance of variance heterogeneity. If insignificant, variance heterogeneity was then removed from the model. Normality of the residuals was examined graphically using a quantile–quantile plot. The significance of each explanatory term introduced in the models was estimated, using non-sequential F statistics (Type III test) and the Kenward–Roger approximation to calculate degrees of freedom. If non-significant, interactive terms were then removed from the model. Otherwise, sliced tests were performed to investigate main effects in the presence of interactive ones. All analyses were implemented in SAS v. 9.3 (SAS Institute, Cary, USA).
Results
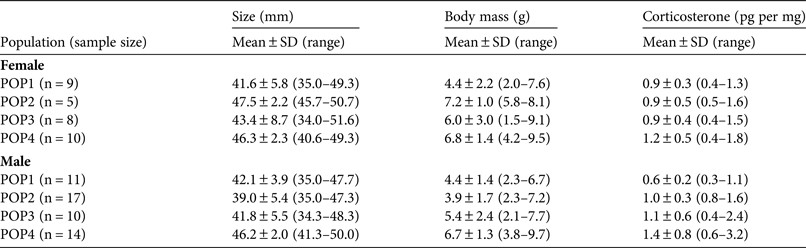
The maximum likelihood ratio test indicated that heterogeneity of variance in body size among populations was significant (
![]() $\chi _{1,3}^2 $
= 27.53, P < 0.001). Body size was marginally influenced by sex (F
1,47.1 = 2.49, P = 0.12; Table 3). The interaction SEX|STATUS also had a marginal effect (F
1,47.1 = 2.20, P = 0.14). Body size varied significantly according to population status (F
1,47.1 = 2.49, P < 0.001). Toads exposed to traffic were smaller than those in control populations (Fig. 3a).
$\chi _{1,3}^2 $
= 27.53, P < 0.001). Body size was marginally influenced by sex (F
1,47.1 = 2.49, P = 0.12; Table 3). The interaction SEX|STATUS also had a marginal effect (F
1,47.1 = 2.20, P = 0.14). Body size varied significantly according to population status (F
1,47.1 = 2.49, P < 0.001). Toads exposed to traffic were smaller than those in control populations (Fig. 3a).
Table 3 Effects of gender (SEX) and population status (STATUS, exposed to intensive vehicle traffic or not) on body size in the yellow-bellied toad, based on an ANOVA F-test type III and least-square mean evaluation for linear combinations, with degrees of freedom, and F, t and P values.
* Degrees of freedom associated with residual variance
The model outputs are in Table 4. The maximum likelihood ratio test indicated that heterogeneity of variance in body condition among populations was significant (
![]() $\chi _{1,3}^2 $
= 8.40, P = 0.04). The interaction SEX|STATUS was not significant (F
1,49.3 = 0.29, P = 0.59). We detected two significant interactions: SVL|STATUS (F
1,53.4 = 8.04, P = 0.006) and SVL|SEX (F
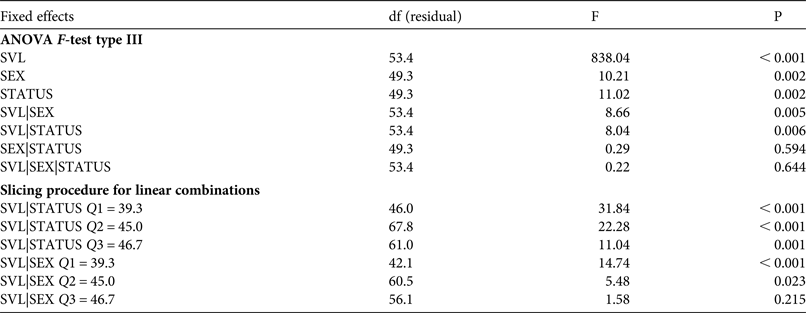
1,53.4 = 8.66, P = 0.005). Our results show that body condition depends on sex and population status (Fig. 2). Females had better body condition than males, regardless of population status. According to the slicing procedure sex-biased body size was significant only for the two first quartiles (Q1 = 39.3 mm, Q2 = 45.0 mm) of the size range (Table 4). Individuals exposed to intensive traffic had an inferior body condition compared to those in control populations. This difference was significant throughout the size range (Table 4).
$\chi _{1,3}^2 $
= 8.40, P = 0.04). The interaction SEX|STATUS was not significant (F
1,49.3 = 0.29, P = 0.59). We detected two significant interactions: SVL|STATUS (F
1,53.4 = 8.04, P = 0.006) and SVL|SEX (F
1,53.4 = 8.66, P = 0.005). Our results show that body condition depends on sex and population status (Fig. 2). Females had better body condition than males, regardless of population status. According to the slicing procedure sex-biased body size was significant only for the two first quartiles (Q1 = 39.3 mm, Q2 = 45.0 mm) of the size range (Table 4). Individuals exposed to intensive traffic had an inferior body condition compared to those in control populations. This difference was significant throughout the size range (Table 4).
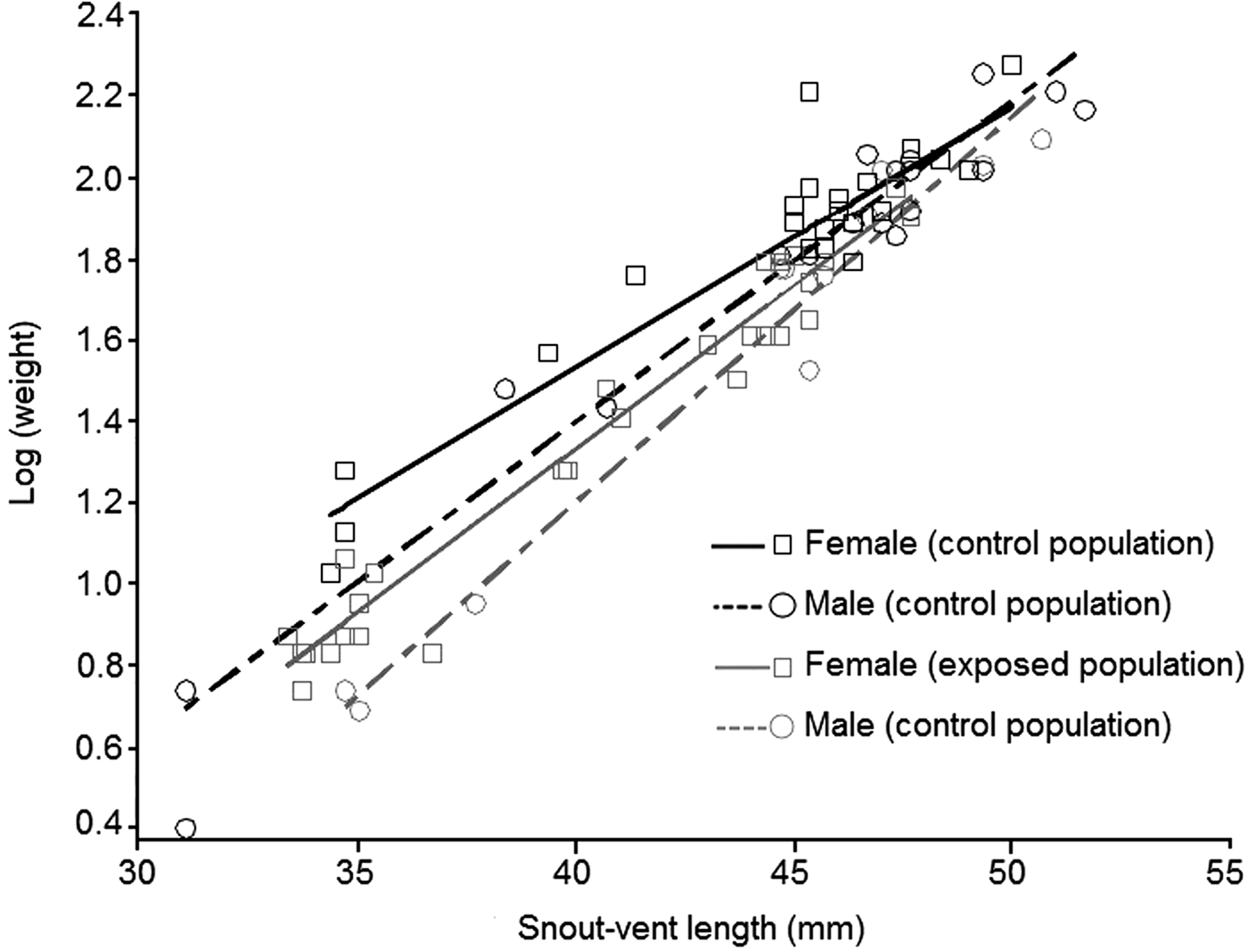
Fig. 2 Relationship between weight (log-transformed) and body size (snout–vent length) in the yellow-bellied toad, according to sex and population status.
Table 4 Effects of gender (SEX) and population status (STATUS, exposed to intensive vehicle traffic or not) on body condition (modelled as linear function between weight and body size, SVL) in the yellow-bellied toad, based on an ANOVA F-test type III and a slicing procedure for linear combinations, with degrees of freedom associated with residual variance, and F and P values.
The maximum likelihood ratio test indicated that heterogeneity of variance in corticosterone concentration among populations was significant (
![]() $\chi _{1,3}^2 $
= 8.75, P = 0.03). Baseline corticosterone concentration was not influenced by sex (F
1,33.2 = 0.42, P = 0.52; Table 5). The interaction SEX|STATUS had a marginal effect (F
1,50.1 = 0.85, P = 0.36). Corticosterone concentration varied according to population status (F
1,31.5 = 5.31, P = 0.02). Individuals of both sexes exposed to intensive traffic had lower corticosterone concentrations than those in control populations (Fig. 3b).
$\chi _{1,3}^2 $
= 8.75, P = 0.03). Baseline corticosterone concentration was not influenced by sex (F
1,33.2 = 0.42, P = 0.52; Table 5). The interaction SEX|STATUS had a marginal effect (F
1,50.1 = 0.85, P = 0.36). Corticosterone concentration varied according to population status (F
1,31.5 = 5.31, P = 0.02). Individuals of both sexes exposed to intensive traffic had lower corticosterone concentrations than those in control populations (Fig. 3b).

Fig. 3 Mean effect (± SE) of intensive vehicle traffic on body size (a) and endocrine stress response (b) in the yellow-bellied toad in exposed populations compared with control populations, based on mixed linear modelling.
Table 5 Effects of gender (SEX) and population status (STATUS, exposed to intensive vehicle traffic or not) on corticosterone concentrations in the yellow-bellied toad, based on an ANOVA F-test type III, with degrees of freedom associated with residual variance, and F and P values.

Discussion
Our results indicate that exposure to intensive vehicle traffic results in a smaller body size in both sexes. The underlying mechanisms are potentially multiple, implicating complex relationships between the toad and its environment, possibly both before and after metamorphosis. During larval ontogeny, changes in tadpole density and/or water temperature in development sites resulting from habitat alteration caused by vehicle traffic on pathways may explain differences in body size at metamorphosis and thereafter at the adult stage (Morand et al., Reference Morand, Joly and Grolet1997). A reduced body size can also result from maternal effects (Dziminski & Roberts, Reference Dziminski and Roberts2006; Wells, Reference Wells2010) through a decrease in the yolk reserves allocated to eggs, which affects embryo development, larval growth and body size at metamorphosis in altered habitats. A smaller adult body size may also reflect increased mortality as a direct result of vehicle traffic (Glista et al., Reference Glista, DeVault and DeWoody2008), as shown in the common frog Rana temporaria (Augert & Joly, Reference Augert and Joly1993). In B. variegata body size plays a critical role in competition between males, affecting mating behaviour and tactics (Seidel, Reference Seidel1999). In females, Rafińska (Reference Rafińska1991) showed a positive correlation between clutch size and body size. Thus, intensive vehicle traffic on unpaved roads could influence sexual selection regimes and reproductive outputs in B. variegata populations.
Our results also show that intensive vehicle traffic results in reduced body condition in both sexes. Poor body condition in exposed populations may result from suboptimal population density triggered by reduced carrying capacities of degraded habitats; for example, alteration of terrestrial habitat can lead to food shortage and nutritional stress by reducing the availability and quality of ground-dwelling insect prey. In amphibians poor body condition usually decreases fitness by negatively influencing annual survival rates (Feder & Burggren, Reference Feder and Burggren1992). Moreover, in females a decrease in body condition as a result of habitat alteration has been linked to a reduction in energy reserves (triglycerides and whole lipids), leading to a decrease in reproductive investment and low fecundity (Scott & Fore, Reference Scott and Fore1995; Homyack & Haas, Reference Homyack and Haas2009). In the yellow-bellied toad, a female's annual breeding decision depends on a combination of internal factors (including energy reserves) and environmental cues (Cayuela et al., Reference Cayuela, Besnard, Bonnaire, Perret, Rivoalen, Miaud and Joly2014). Accordingly, a reduction in energy reserves caused by exposure to vehicle traffic has a detrimental effect on female fecundity.
We found that corticosterone production is lower in populations exposed to intensive vehicle traffic than in control populations. We cannot rule out the possibility that this result may be attributable to the low number of populations sampled and/or the absence of stress-induced measurements. Furthermore, as glucocorticoid production constitutes an immediate response to an environmental stressor, it is conceivable that the observed pattern may be the result of stress responses to one or several factors other than the intensity of vehicle traffic. We suggest that good quality habitat may favour increased sexual activity (male–male competition and female harassment by males), enhancing corticosterone production. Unlike in birds and mammals, it seems that the inhibition effects of glucocorticoids on the reproductive system, through the suppression of gonadal hormone secretion, is equivocal in amphibians (Narayan, Reference Narayan2013). In the cane toad Rhinella marina, for example, after stress induction, corticosterone production covaries positively with testosterone secretion when toads are sexually active, whereas an inverse relationship is reported in non-breeding toads (Narayan et al., Reference Narayan, Hero and Cockrem2012a,Reference Narayan, Molinia, Cockrem and Herob). In Woodhouse's toads Bufo woodhousii there is a positive correlation between sexual activity (vocal effort) and circulating corticosterone levels although corticosterone production is not related to the production of sexual steroids (Leary et al., Reference Leary, Garcia, Knapp and Hawkins2008). These examples illustrate the complexity of the relationships between the reproductive endocrine system (the hypothalamo–pituitary–gonadal axis), the stress endocrine system (the hypothalamo–pituitary–interrenal axis) and amphibian behaviour. Thus, despite the current trend for physiological conservation and the use of glucocorticoids as bio-indicators in amphibians (Narayan, Reference Narayan2013), the observed patterns should be considered with caution and preferentially in combination with other biological indices (e.g. morphological).
Conservation recommendations
We suggest that the intensity of vehicle traffic should be limited during the breeding season of the yellow-bellied toad (April–September). We consider two scenarios: (1) traffic does not affect economic activities directly (e.g. use of non-essential unpaved roads by individuals for leisure activity) and can be avoided during the breeding season; (2) traffic directly affects economic activities (e.g. use of essential unpaved roads for forestry or quarry activities) and cannot be avoided. In the first case we propose the exclusion of traffic from aquatic habitats and a 500 m diameter buffer zone around these habitats (Hartel, Reference Hartel2008) during the breeding season, to avoid deleterious effects on recruitment and adult survival. Traffic may be authorized during October–March. In the second case we encourage multilateral conventions between environmental protection organizations or governmental agencies for nature conservation and territorial authorities or professionals such as mining operators. The conditional use of roads, as well as any quarry exploitation plan, should be adapted, taking into account the specificity of local situations and contexts; for example, breeding sites should be protected from traffic by creating alternative pathways, which should be stabilized and maintained using rockfill to avoid rut creation. During the non-breeding season (October–March) the use of pathways should not be restricted to avoid the natural silting up of existing breeding sites and to ensure the creation of new ones. If this is not possible, substitute breeding sites could be created away from the pathways used for sustaining essential economic activities. These recommendations are under negotiation with the mining operator at one of the two study sites exposed to vehicle traffic. Further investigation will be required to evaluate and compare the effectiveness of these management recommendations.
Acknowledgements
This research was funded by the Société des Carrières du Bourget du Lac, whose partners Jerome Langain and Regis Chevallier have been involved in the conservation of the yellow-bellied toad for almost 10 years. Capture of toads was authorized by the Conseil Scientifique Régional du Patrimoine Naturel of Rhône-Alpes (REMIPP-13-BRM-229–RD).
Biographical sketches
Hugo Cayuela conducts research on evolution and conservation biology, and is particularly interested in amphibians. Ludivine Quay works on amphibian and reptile conservation. Adeline Dumet is a laboratory technician interested in ecophysiological and genetic analyses. Jean-Paul Léna's research interests include dispersal ecology and evolution, sexual selection and population dynamics. Claude Miaud's research interests lie in amphibian ecology and conservation. Vincent Rivière is a conservationist specializing in ecological restoration and the ecological management of industrial projects.










