Introduction
Habitat alteration and destruction are major causes of species extinction (Tilman et al., Reference Tilman, May, Lehman and Nowak1994). The seven species of sea turtle are threatened by development, artificial lighting and tourism on their nesting beaches (Sella & Fuentes, Reference Sella and Fuentes2019), and fisheries bycatch has resulted in drastic declines of some sea turtle populations (Santos et al., Reference Santos, Coelho, Fernandez-Carvalho and Amorim2013; Hays et al., Reference Hays, Bailey, Bograd, Bowen, Campagna and Carmichael2019). Additional threats are the harvest of eggs and adults on nesting beaches (McClenachan et al., Reference McClenachan, Jackson and Newman2006; Polasky, Reference Polasky2008), and anthropogenic pressure in the form of climate change, land transformation and pollution (Patino-Martinez et al., Reference Patino-Martinez, Marco, Quinones and Hawkes2012; Patino-Martinez et al., Reference Patino-Martinez, Godley, Quiñones and Marco2017; Maxwell et al., Reference Maxwell, Broderick, Dutton, Fossette-Halot, Fuentes and Reina2019; Patrício et al., Reference Patrício, Varela, Barbosa, Broderick, Catry and Hawkes2019; Sage, Reference Sage2020). The decline of sea turtles has generated research and conservation interest from universities, government agencies, NGOs and the public (Hamann et al., Reference Hamann, Godfrey, Seminoff, Arthur, Barata and Bjorndal2010; Cornwell & Campbell, Reference Cornwell and Campbell2012; Godley et al., Reference Godley, Broderick, Colman, Formia, Godfrey and Hamann2020), but information on sea turtle populations that nest in relatively undisturbed areas is limited (Tomas et al., Reference Tomas, Godley, Castroviejo and Raga2010; Mazaris et al., Reference Mazaris, Schofield, Gkazinou, Almpanidou and Hays2017).
The loggerhead sea turtle Caretta caretta nests in tropical and subtropical regions (Baldwin et al., Reference Baldwin, Hughes, Prince, Bolten and Witherington2003; Ehrhart et al., Reference Ehrhart, Bagley, Redfoot, Bolten and Witherington2003). Population trends are variable, depending on the levels of threats and/or protection: some populations are increasing (Casale & Matsuzawa, Reference Casale and Matsuzawa2015) and others decreasing (Casale, Reference Casale2015), and for some there are insufficient data (Nordberg et al., Reference Nordberg, Macdonald, Zimny, Hoskins, Zimny and Somaweera2019). The largest known rookeries are in the north-west Atlantic in Florida, USA, and in Oman in the north-west Indian Ocean (Casale & Tucker, Reference Casale and Tucker2017). The Republic of Cabo Verde in West Africa, an archipelago of 10 islands, has been considered the third largest loggerhead turtle nesting subpopulation (Casale & Marco, Reference Casale and Marco2015). The species is categorized as Vulnerable on the IUCN Red List, but with the north-east Atlantic nesting colony (nesting primarily in Cabo Verde) categorized as Endangered (Casale & Marco, Reference Casale and Marco2015). The latter has been identified as an isolated subpopulation, based on molecular studies (Monzon-Arguello et al., Reference Monzon-Arguello, Rico, Naro-Maciel, Varo-Cruz, Lopez, Marco and Felipe Lopez-Jurado2010; Stiebens et al., Reference Stiebens, Merino, Roder, Chain, Lee and Eizaguirre2013), and is managed as a Regional Management Unit (Wallace et al., Reference Wallace, DiMatteo, Hurley, Finkbeiner, Bolten and Chaloupka2010).
In Cabo Verde the loggerhead turtle is threatened by high levels of anthropogenic development in nesting habitats (Loureiro, Reference Loureiro2008; Abella Perez et al., Reference Abella Perez, Marco, Martins and Hawkes2016) and illegal capture for the meat trade and local consumption (5–36% of nesting females per year; Marco et al., Reference Marco, Abella, Liria-Loza, Martins, Lopez and Jimenez-Bordon2012; Dutra & Koenen, Reference Dutra and Koenen2014; Hancock et al., Reference Hancock, Furtado, Merino, Godley and Nuno2017). Additional threats are fisheries bycatch (Lewison et al., Reference Lewison, Crowder, Wallace, Moore, Cox and Zydelis2014; Coelho et al., Reference Coelho, Santos, Fernandez-Carvalho and Amorim2015; Lopes et al., Reference Lopes, Passos, Rodrigues, Koenen, Stiebens, Székely and Dutra2016; Bielli et al., Reference Bielli, Alfaro-Shigueto, Doherty, Godley, Ortiz and Pasara2019) and light pollution along the coast (Silva et al., Reference Silva, Marco, Da Graça, Pérez, Abella and Patino-Martinez2017). It has been estimated that 75–85% of all nesting activity in Cabo Verde is on the island of Boa Vista (Marco et al., Reference Marco, Da Graça, García-Cerdá, Abella and Freitas2015; Tanner et al., Reference Tanner, Marco, Martins, Perez and Hawkes2019), where most research and monitoring efforts have focused since 2000 (López-Jurado et al., Reference López-Jurado, Varo-Cruz and López-Suárez2003; Hawkes et al., Reference Hawkes, Broderick, Coyne, Godfrey, Lopez-Jurado and Lopez-Suarez2006; Monzon-Arguello et al., Reference Monzon-Arguello, Rico, Naro-Maciel, Varo-Cruz, Lopez, Marco and Felipe Lopez-Jurado2010; Camacho et al., Reference Camacho, Boada, Oros, Lopez, Zumbado, Almeida-Gonzalez and Luzardo2013; Scott et al., Reference Scott, Biastoch, Roder, Stiebens and Eizaguirre2014; Abella Perez et al., Reference Abella Perez, Marco, Martins and Hawkes2016; Usategui-Martín et al., Reference Usategui-Martín, Liria-Loza, Miller, Medina-Suárez, Jiménez-Bordón, Pérez-Mellado and Montero2019). Information about the abundance, trends and reproductive success of nesting females on other islands is limited (Taylor & Cozens, Reference Taylor and Cozens2010; Cozens et al., Reference Cozens, Taylor and Gouveia2011; Dutra & Koenen, Reference Dutra and Koenen2014; Rocha et al., Reference Rocha, Melo, Rebelo and Catry2015; Laloë et al., Reference Laloë, Cozens, Renom, Taxonera and Hays2019), but such data, particularly long time series, are critical for the conservation of this subpopulation (Wallace et al., Reference Wallace, DiMatteo, Hurley, Finkbeiner, Bolten and Chaloupka2010; Mazaris et al., Reference Mazaris, Schofield, Gkazinou, Almpanidou and Hays2017).
Here we present the first detailed study of loggerhead turtle nesting on Maio over 4 years, including abundance, spatial distribution of nesting, reproductive success and threats. We also evaluated hatchling productivity (the number of hatchlings that would reach the sea) under two nest management strategies that were implemented on beaches with low natural hatching success. Our findings will facilitate a status assessment of this subpopulation and inform management actions.
Study area
The 269 km2 island of Maio (Fig. 1) is one of the 10 islands in Cabo Verde, West Africa. Loggerhead turtles nest on 38 km of sandy beaches along its 110 km coastline. The high-energy, steep beaches of Maio are largely undeveloped, with near pristine habitat and little anthropogenic disturbance. The colour and grain size of the sand varies. Only one of the beaches (Beach Rotxa, 0.8 km long) is artificially illuminated (Fig. 1).
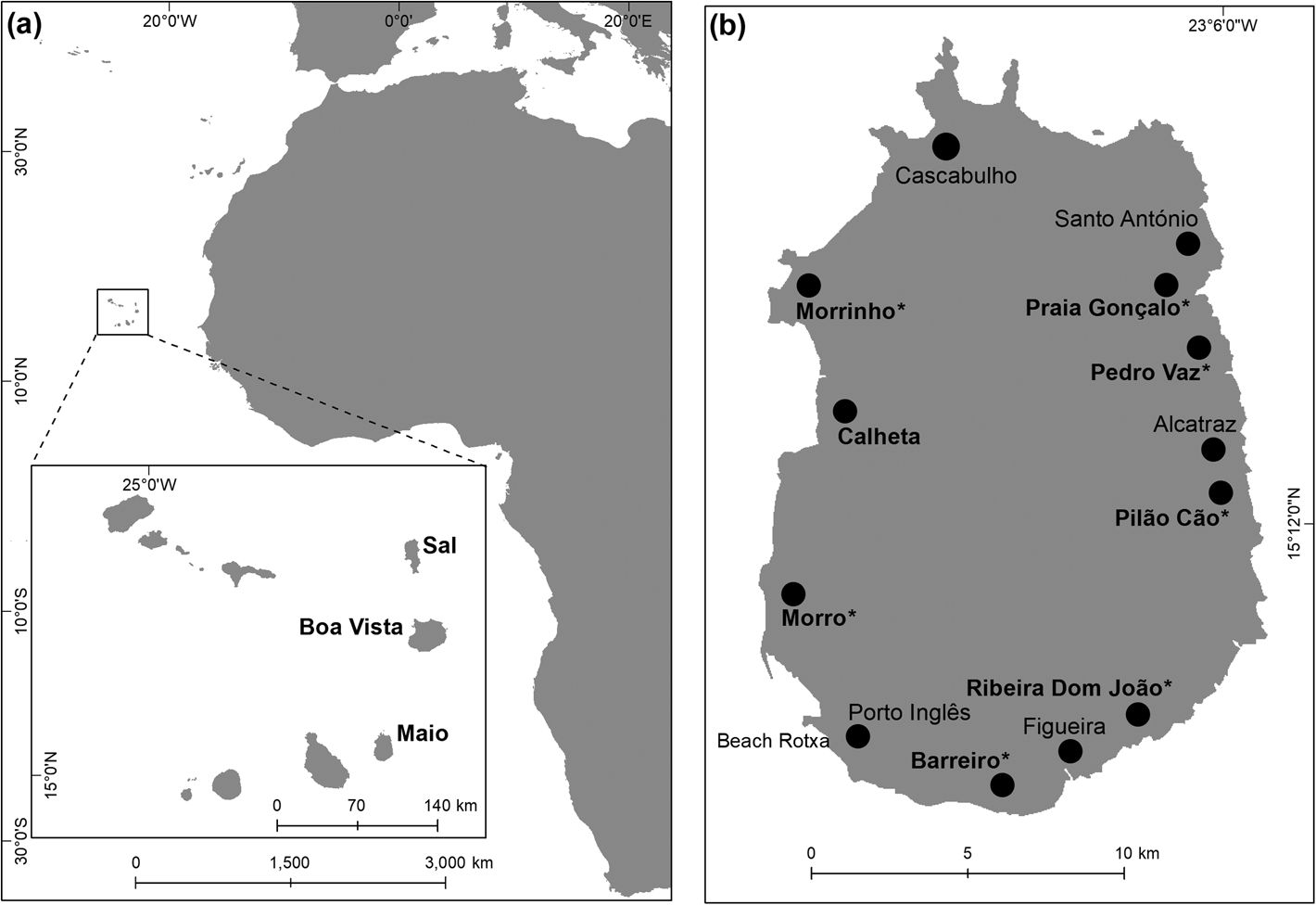
Fig. 1 (a) Location of the Cabo Verde archipelago off West Africa. (b) Maio Island, with 13 study camps that were established to monitor all of the island's loggerhead sea turtle Caretta caretta nesting beaches. The sites in bold are in Table 1, and we examined clutch losses and effect of different nest management strategies at sites marked with * (Tables 2 & 3). Beach Rotxa is the only site with artificial illumination.
Table 1 Survey data of loggerhead sea turtle Caretta caretta nesting at the study sites (length of beach at each site in parentheses) on Maio Island, Cabo Verde during 2016–2019.

Table 2 Estimated loss and survival of clutches and hatching success at study the sites on Maio Island where we assessed reproductive success quantitatively, in 2017 and 2018.
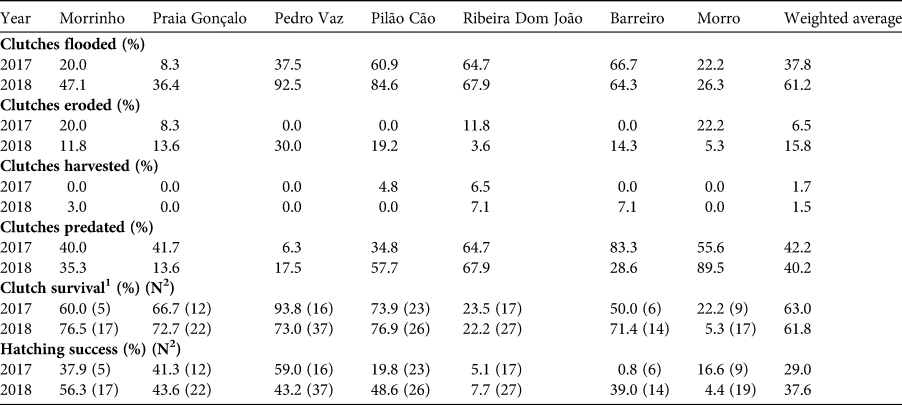
1 Clutch survival is defined as at least one hatchling emerging from the clutch.
2 N, number of clutches assessed.
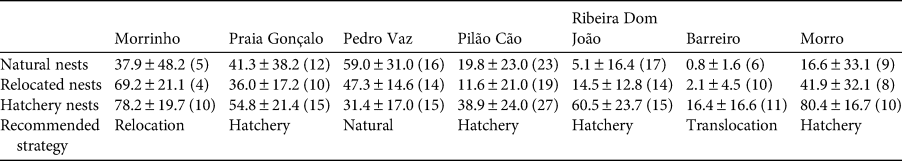
Table 3 Hatching success (% ± SD, with number of nests in parentheses) for different nest management strategies, and recommended strategy for each site where we assessed reproductive success quantitatively.
Methods
Nesting activity
The entire 38 km of sandy beaches were surveyed for nesting abundance by 44 fieldwork teams (2–4 people per team). Beaches were monitored daily for 110–130 nights per nesting season, during 20.00–6.00, for 4 consecutive years (2016–2019). The teams recorded locations of all nesting activities (clutches laid and aborted nesting attempts) observed overnight, using GPS devices, and erased all turtle tracks using wooden rakes. Any nesting activity not recorded during the night was mapped during a daily track count in the early morning. We calculated the proportion of nesting activities resulting in successful deposition of eggs (nesting success) and nest density per linear km of beach for each site.
Hatching success
We determined the geographical coordinates of 88 clutches in 2017 and 162 in 2018, marking their positions with wooden stakes. For each clutch we counted the number of eggs during oviposition and monitored the nest until hatching. We determined hatching success as the per cent of eggs producing hatchlings. We recorded visible impacts on nests such as beach erosion, tidal flooding, natural predation and illegal harvesting at seven sites during 2017 and 2018 (Fig. 1, Table 2).
Nest management strategies
To evaluate nest management strategies that can help mitigate natural or anthropogenic threats, we conducted a field experiment in two phases. The first phase, in 2017, included seven study sites (Fig. 1), with 182 clutches subjected to nest management (either hatchery or relocation) and 88 nests remaining in situ as controls. In the hatchery treatment, we transferred 103 clutches to seven experimental open beach hatcheries (enclosures surrounded by a 1 m high plastic mesh and wooden fence to avoid predators such as crabs and dogs; 7 × 7 m; on a 0° slope, above the high tide line; one hatchery in each of the seven study areas; Fig. 1), with 11–15 nests per hatchery. In the relocation treatment, we moved 79 clutches (10–19 per site) to areas well beyond the high tide line, to avoid flooding of nests. Control nests were not manipulated but observed in their original locations. We buried all treatment clutches at a maximum depth of 45 cm, in nest chambers with a horizontal diameter of 25 cm, which corresponds to the dimensions of natural nests in this area. Whenever possible, we randomly selected three nests per night (or on consecutive nights) at each site, one for each treatment and the control, so that the incubation regime of the study groups coincided in time. We counted the eggs and determined the hatching success for each treatment and control nest at each site.
In the second phase, in 2018, based on our experience in 2017, we chose one of the following four strategies for the seven sites (414 nests studied; Fig. 1, Table 3): (1) leaving nests in situ (site 3), (2) relocation to a hatchery on the same beach (sites 2, 4, 5, 7), (3) relocation to an area beyond the flood line on the same beach (site 1), and (4) translocation to a hatchery on a different beach (site 6). We calculated mean hatching success for each strategy and used this to generate a theoretical estimate of the hatchling productivity:
where H prod is the hatchling productivity, N the number of nests of each treatment, M e the mean number of eggs per nest and HSme the mean hatching success of each management strategy.
Statistical analyses
We used R 3.5.2 (R Core Team, 2018) for statistical analyses. Normality and heteroscedasticity were tested using Shapiro–Wilk and Levene's tests, respectively. We tested if nesting success and hatching success were significantly different between years and between sites using a one-way and a two-way ANOVA, respectively. If significant differences were detected, we used a multiple comparisons Tukey test, to determine which years or sites were different. We performed a χ² test for given probabilities, to identify any significant differences in flooding and predation between years and sites. We tested if management strategies were significantly different using a Kruskal–Wallis test.
Results
Numbers and spatial-temporal distribution of nests
In each year we recorded the first nests in early June, and the nesting season continued until early November, with peak nesting activity in August (Fig. 2). We observed a total of 10,985 nesting activities (clutches laid and aborted nesting attempts) in 2016, 12,806 in 2017, 30,075 in 2018 and 19,316 in 2019, corresponding to a minimum of 4,063, 5,429, 14,364 and 7,937 clutches, respectively (Table 1). The eastern coast of Maio (sites 2, 3 and 4; Fig. 1) had the highest number and density of nests every year (1,281–2,113 nests/km; Table 1) and the north-west had the lowest density (49–151 nests/km; Table 1).
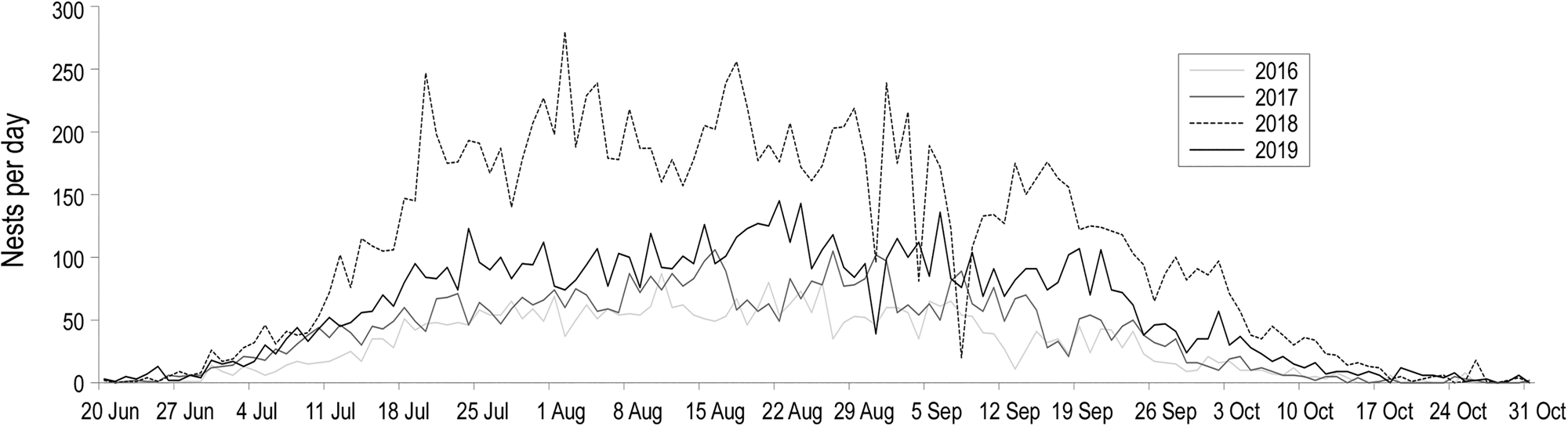
Fig. 2 Temporal pattern of loggerhead turtle nesting on Maio, Cabo Verde.
On the island of Boa Vista in Cabo Verde, females produce 3–5 clutches per season (Varo-Cruz, Reference Varo-Cruz2010). Based on this, there could have been 813–4,788 nesting females per year throughout Maio during the study period. Assuming a mean of four clutches per female and season, we estimate there were 1,016, 1,357, 3,591 and 1,984 nesting females during the four years of study, respectively.
Reproductive success
Nesting success varied between years, being lowest (37.0%) in 2016 and highest (47.8%) in 2018 (P > 0.05). It also varied between sites, being consistently lowest (20.2–30.8%) at site 5 and highest (48.1–65.0%) at site 2 (ANOVA df = 8, F = 13.53, P < 0.0001; Table 2). Hatching success was significantly different between sites but not between years (two-way ANOVA site: year df = 6, F = 3.34, P < 0.001). Hatching success was lowest (minimum 4.4%) at sites 5 and 7, and highest (maximum 59.9%) at sites 2 and 3, which had the highest abundance and density of nests (Table 2).
Nest flooding varied between years (χ 2 = 11.07, df = 1, P < 0.001) and sites, from 8.3% of nests flooded at site 2 in 2017 to 92.5% at site 3 in 2018 (χ 2 = 43.71, df = 6, P < 0.0001; Table 2). Nest poaching was confirmed at two sites in 2017 and at three sites in 2018; an estimated 1.7% (n = 171 in 2017) and 1.5% (n = 178 in 2018) of clutches were illegally harvested. Natural predation, mainly by ghost crabs Ocypode sp. (Marco et al., Reference Marco, Da Graça, García-Cerdá, Abella and Freitas2015; Rodrigues et al., Reference Rodrigues, Freitas, Delgado and Soares-Gomes2016) was stable between years for the entire island (from 40.2% in 2018 to 42.2% in 2017; P > 0.05), but varied between beaches (χ 2 = 49.69, df = 6, P < 0.0001). Nest predation by domestic dogs was documented for the first time on the island, at site 7, near the city of Porto Inglês (Fig. 1), the largest settlement on the island. Of all nests there, 68.4% were partially or totally affected.
Nest management strategies
Hatching success was significantly higher in hatcheries compared to natural or relocated nests, considering site and year as random variables (mixed effect model F = 136.74, P < 0.0001). The expected productivity of hatchlings increased in hatcheries: 395,693 hatchlings in hatcheries vs 201,492 hatchlings in control nests. Relocated nests (2018, site 1; Fig. 1) had a slightly higher hatching success than in situ control nests (70% relocated vs 56% control; P > 0.05) and hatchling productivity was 51,535 hatchlings in relocated nests vs 41,653 in control nests. Hatching success for nests moved to a hatchery on a different beach (from site 6; Fig. 1, Table 3) was also significantly higher than that of control nests (88% translocated vs 39% control; Kruskal–Wallis test χ 2 = 16,533, df = 1, P < 0.0001) and hatchling productivity was 81,648 hatchlings in translocated nests vs 36,136 in control nests.
Discussion
Importance of Maio for nesting loggerhead turtles
This is the first multi-year quantitative report on the nesting activity of loggerhead turtles on Maio Island, Cabo Verde. Our findings demonstrate that the nesting colony is considerably larger than previously estimated (Cozens et al., Reference Cozens, Taylor and Gouveia2011; Dutra & Koenen, Reference Dutra and Koenen2014). In 2018, there were c. 15,000 nests on Maio, which is 7.5% of the estimated global total of 200,000 nests/year (Casale & Tucker, Reference Casale and Tucker2017). Our counts on Maio and data from other locations (Broderick et al., Reference Broderick, Glen, Godley and Hays2002; Marcovaldi & Chaloupka, Reference Marcovaldi and Chaloupka2007; Zbinden et al., Reference Zbinden, Largiader, Leippert, Margaritoulis and Arlettaz2007; Durmus et al., Reference Durmus, Ilgaz, Ozdemir and Yerli2011; Casale & Tucker, Reference Casale and Tucker2017; Laloë et al., Reference Laloë, Cozens, Renom, Taxonera and Hays2019) indicate Maio is among the five largest loggerhead nesting colonies globally (Table 4), making it an important site for loggerhead conservation both regionally and globally (Wallace et al., Reference Wallace, DiMatteo, Hurley, Finkbeiner, Bolten and Chaloupka2010, Reference Wallace, DiMatteo, Bolten, Chaloupka, Hutchinson and Abreu-Grobois2011; Mazaris et al., Reference Mazaris, Schofield, Gkazinou, Almpanidou and Hays2017).
Table 4 Loggerhead sea turtle nesting on Maio Island compared to major subpopulations around the world.
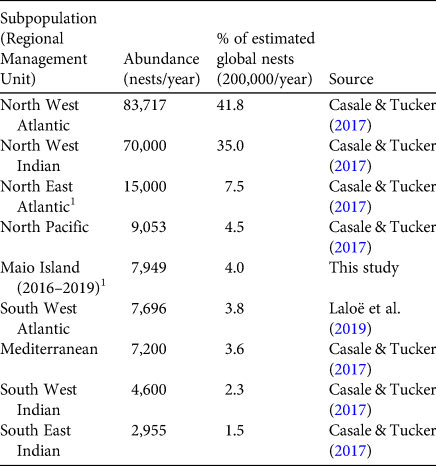
1 Data from Cabo Verde, subpopulation of the North East Atlantic Regional Management Unit.
Nest mortality and management strategies
The most prevalent threats to the nests on Maio were tidal flooding, beach erosion and predation by crabs. Near the city of Porto Inglês, predation by dogs is an emerging problem that needs to be addressed. Poaching was not a major threat on Maio, probably as a result of ongoing conservation efforts. The eastern coast hosts the largest number of nests at the highest density, with the highest nesting success and consequently the greatest number of hatchlings. This could be positively reinforced over time if nesting philopatry is restricted to the beach or area where hatchlings emerged. In situ nesting success varied between beaches. Females nested more frequently on the beaches with higher hatching success (sites 2 and 5; Table 1). This could confer an adaptive advantage if the females’ preferred microhabitats increase the chances of survival for their offspring (Marco et al., Reference Marco, Abella, Martins, López and Patino-Martinez2018a; Patrício et al., Reference Patrício, Varela, Barbosa, Broderick, Airaud and Godley2018).
Environmental conditions and mortality rates vary between areas. To increase hatchling productivity, different nest management strategies are thus required, depending on the local context. On Maio, the productivity of hatchlings from nests in suboptimal areas could be increased by up to 2.3 times by translocating clutches to protected beaches with more favourable conditions. Hatchling productivity doubled for clutches moved to hatcheries on the same beach where they were laid. At sites with high natural hatching success, we recommend relocating clutches to areas beyond the flood line on the same beach (without hatcheries) or leaving the nests in situ. Although we found hatcheries to be successful, they also require greater financial investment and effort, and are therefore not always the best management option (Sieg et al., Reference Sieg, Binckley, Wallace, Santidrian Tomillo, Reina, Paladino and Spotila2011; van de Merwe et al., Reference van de Merwe, Ibrahim and Whittier2013). Use of hatcheries for conservation purposes should be considered only as a last resort if the population is threatened, there is high nest mortality and nests cannot be managed in situ (Patino-Martinez et al., Reference Patino-Martinez, Marco, Quinones and Hawkes2012). In addition, sand temperature should be recorded in hatcheries and more widely on nesting beaches, to generate robust estimates of primary sex ratios, a global research priority for sea turtles (Hamann et al., Reference Hamann, Godfrey, Seminoff, Arthur, Barata and Bjorndal2010).
Conservation on Maio
The island of Maio has pristine beaches without light pollution, extensive coastal infrastructure or mass tourism, and with high numbers and densities of loggerhead turtle nests. Given that coastlines globally are becoming increasingly urbanized, illuminated and disturbed (Godoy & Stockin, Reference Godoy and Stockin2018; Windle et al., Reference Windle, Hooley and Johnston2018; Blackburn et al., Reference Blackburn, Pelling, Marques, Wolanski, Day, Elliot and Ramachandran2019; Winger et al., Reference Winger, Weeks, Farnsworth, Jones, Hennen and Willard2019), and that sea turtles are considered conservation-dependent (Godfrey & Godley, Reference Godfrey and Godley2008), undisturbed rookeries such as Maio are important refuges for conservation. The protection of these unique habitats must be prioritized to conserve coastal biodiversity (Antworth et al., Reference Antworth, Pike and Stiner2006). Conditions on Maio could deteriorate if, as has been the case on other islands in Cabo Verde, tourism and related infrastructure are developed along the coast.
For Maio, the main conservation objectives are to maintain the natural conditions of the coastal ecosystem, to train and involve local people in the sustainable management of their natural resources, and to improve peoples’ well-being through social investment. Sea turtle nesting beaches in other Cabo Verdean islands (Silva et al., Reference Silva, Marco, Da Graça, Pérez, Abella and Patino-Martinez2017; Laloë et al., Reference Laloë, Cozens, Renom, Taxonera and Hays2019) and beyond (Godoy & Stockin, Reference Godoy and Stockin2018; Blackburn et al., Reference Blackburn, Pelling, Marques, Wolanski, Day, Elliot and Ramachandran2019; Colman et al., Reference Colman, Lara, Bennie, Broderick, de Freitas and Marcondes2020) are increasingly urbanized, illuminated and exploited for tourism. Any future development on Maio will need to consider our findings, and decisions should be based on a multi-criteria decision analysis for nature conservation (Esmail & Geneletti, Reference Esmail and Geneletti2018).
Importance of Cabo Verde
Our observations on Maio during 2016–2019 and the increase in nesting activity in Cabo Verde since 2015 (Marco et al., Reference Marco, Tavares Martins, Abella and Patino-Martinez2018b; Laloë et al., Reference Laloë, Cozens, Renom, Taxonera and Hays2019), highlight the importance of the Cabo Verde subpopulation for loggerhead turtle conservation. The annual number of loggerhead turtle nests on Sal Island (Laloë et al., Reference Laloë, Cozens, Renom, Taxonera and Hays2019) is similar to that of Maio. Approximately 75% of nesting in Cabo Verde occurs on the island of Boa Vista (Marco et al., Reference Marco, Abella, Liria-Loza, Martins, Lopez and Jimenez-Bordon2012, Reference Marco, Da Graça, García-Cerdá, Abella and Freitas2015; Tanner et al., Reference Tanner, Marco, Martins, Perez and Hawkes2019), Maio and Sal islands host 16.6% of nests (Laloë et al., Reference Laloë, Cozens, Renom, Taxonera and Hays2019) and the remainder are deposited on the other islands (Ministério da Agricultura e Ambiente, Direçao Nacional do Ambiente, unpubl. data). We thus estimate there have been a mean of 95,762 ± SD 55,038 nests annually in Cabo Verde during 2016–2019, which may be the largest loggerhead turtle nesting population globally (Florida, western North Atlantic: 83,717 nests/year, Oman: 70,000 nests/year; Casale & Tucker, Reference Casale and Tucker2017). The species may be increasing in abundance in part of its range and decreasing elsewhere (Wallace et al., Reference Wallace, DiMatteo, Hurley, Finkbeiner, Bolten and Chaloupka2010). There has been evidence of a decline in the number of loggerhead turtle nests in Oman during 1978–2016 (55,202 nests/year), possibly because of a decreasing number of females (Willson et al., Reference Willson, Witherington, Baldwin, Tiwari, Al Sariri and Al Kiyumi2020), and the 30-year pattern (1989–2018) of the population in Florida portrayed a general non-monotonic trend with wide fluctuations (Ceriani et al., Reference Ceriani, Casale, Brost, Leone and Witherington2019; Witherington et al., Reference Witherington, Kubilis, Brost and Meylan2009). To compare population trends and assess the overall status of the species (Richards et al., Reference Richards, Epperly, Heppell, King, Sasso and Moncada2011; Mazaris et al., Reference Mazaris, Schofield, Gkazinou, Almpanidou and Hays2017), it is necessary to accurately estimate both the number of nests and the reproductive parameters that allow the number of adult females to be calculated (Esteban et al., Reference Esteban, Mortimer and Hays2017; Ceriani et al., Reference Ceriani, Casale, Brost, Leone and Witherington2019). In Cabo Verde, further studies are required to examine whether nesting activity was unusually high during 2016–2019, or whether our data reflect the normal pattern for this population.
The island of Maio is a globally important refuge for loggerhead turtle reproduction. Its coastal habitats are largely undisturbed, and other species of sea turtle, or taxa belonging to so-called dark diversity (Lewis et al., Reference Lewis, de Bello, Bennett, Fibich, Finerty and Götzenberger2017), may be present. However, there is pressure for economic growth from its inhabitants. The authorities have initiated the expansion of the island's port and plan to build additional tourist accommodation, which could result in an increase in the number of inhabitants and associated pollution. In this scenario, conservation problems involve socio-cultural, economic, and spatially explicit factors. Natural science alone is insufficient to find solutions to complex conservation problems that have social dimensions (Sandbrook et al., Reference Sandbrook, Adams, Büscher and Vira2013) and therefore, appropriate methods of stakeholder engagement and synthesis of their knowledge and interests are needed (Pullin et al., Reference Pullin, Frampton, Jongman, Kohl, Livoreil and Lux2016). Communities must take ownership of the management and conservation of their natural resources (Rees et al., Reference Rees, Alfaro-Shigueto, Barata, Bjorndal, Bolten and Bourjea2016; Patino-Martinez et al., Reference Patino-Martinez, Dos Passos, Dos Reis and Moreno2020) and benefit from the economic investment derived from conservation projects.
Acknowledgements
We thank local rangers, team leaders, supervisors and volunteers for their help with data collection; the Cabo Verde national environmental authority, Direção Nacional do Ambiente, for authorizing the study; MAVA Fondation pour la Nature, the National Oceanic and Atmospheric Administration and the U.S. Fish & Wildlife Service for funding; Gemma Charles, Lucy Hawkes, Graeme Hays and an anonymous reviewer for their help in improving the manuscript.
Author contributions
Conceptualization: JP-M, LDP, AT, MT; methodology: JP-M, LDP; data analysis: JP-M, IOA, TS; visualization: JP-M, IOA, MT, TS, RM; supervision: JP-M; project administration: JP-M, LDP, RM; resources: LDP, AT, MT, TS; funding acquisition: LDP, AT, MT; writing: JP-M; revisions: IOA, MT, TS, RM.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards. It did not involve human subjects and/or collection of specimens. The research followed protocols recommended by Stokes et al. (Reference Stokes, Epperly, Avens, Belskis, Benson and Braun-McNeill2008). The national environmental authority Direção Nacional do Ambiente provided permits for this research.








