The brown hare Lepus europaeus is an important small game species in Europe (Pielowski, Reference Pielowski, Pielowski and Pucek1976). It is thought to have evolved on the open steppe grasslands of Eurasia and has adapted to mixed arable agriculture (Frylestam, Reference Frylestam1980). European hare hunting bags indicate a dramatic decline during 1960-1980 (Smith et al., Reference Smith, Vaughan Jennings and Harris2005). Changes in agricultural management, heavy hunting pressure and diseases are the most likely factors responsible for this long-term decline. As a consequence, although the brown hare is not on the IUCN Red List (IUCN, 2006), it is protected under Appendix III of the Convention of the Conservation of European Wildlife and Natural Habitats (Bern Convention; Smith et al., Reference Smith, Vaughan Jennings and Harris2005), and is classified as a ‘priority species of conservation concern’ by the UK government (Smith et al., Reference Smith, Vaughan Jennings and Harris2005).
To stabilize population declines, especially as a result of previously heavy hunting pressure, restocking programmes using allochthonous individuals have been carried out in several European countries. While simple in concept, restocking as a management tool remains controversial (Booth, Reference Booth1988; Conant, Reference Conant1988; Griffith et al., Reference Griffith, Scott, Carpenter and Reed1989; Kleiman, Reference Kleiman1989; Storfer, Reference Storfer1998). Criticisms focus on the lack of long-term quantitative information on post-release impact (Scott & Carpenter, Reference Scott and Carpenter1987), difficulty of establishing success or failure criteria (Seddon, Reference Seddon1999), and concerns that extensive gene flow can interfere with local adaptations (Storfer, Reference Storfer1998).
Breeding stations in Bulgaria, Slovakia, Hungary and Poland have traditionally functioned as source populations for restocking operations in Central and Western Europe. These practices may have affected the historical distribution and genetic integrity of indigenous hare species in several European countries (Thulin et al., Reference Thulin, Jaarola and Tegelstrom1997; Pierpaoli et al., Reference Pierpaoli, Riga, Trocchi and Randi1999). In Greece, the brown hare is still legally hunted but restocking programmes were abandoned from 2001. The records of the Ministry of Agriculture and of hunting associations show that 2,000 reared individuals (bought from private Greek breeding stations but previously imported mainly from Italy, Yugoslavia and Bulgaria) were released during 1991-2001. However, until 1998, restocking operations were uncontrolled and there was no monitoring of released animals.
In a previous study (Mamuris et al., Reference Mamuris, Sfougaris and Stamatis2001) we examined wild brown hares from Greece collected during the hunting seasons of 1998 and 1999, and reared brown hares from two farms in 1999. Extensive restriction fragment length polymorphism (RFLP) analysis of three different mitochondrial DNA (mtDNA) segments revealed three groups of individuals: (1) reared brown hares, (2) wild brown hares, and (3) wild individuals with mtDNA haplotypes closely related, but not identical, to those observed in the reared group of hares (Mamuris et al., Reference Mamuris, Sfougaris and Stamatis2001). To trace the origins of the reared individuals and determine the degree of genetic introgression of released brown hares into existing populations in 1999-2004 we collected 187 brown hare samples from Epirus and Thessaly, 42 from north Greece, 53 from north-east Greece, 28 from south Greece and eight from Crete (Fig. 1). The samples were either from hunted hares or from dead individuals found in the wild. We also collected a total of 323 samples from France, the Netherlands, Germany, Poland, Austria, Switzerland, Serbia and Bulgaria, and 60 samples from Turkey and Israel (Fig. 1). Based on Mamuris et al. (Reference Mamuris, Sfougaris and Stamatis2001) we identified diagnostic RFLP markers on the mtDNA that could rapidly and unambiguously differentiate the three previously defined groups of brown hare mtDNA haplotypes: a segment of the cytochrome b (Cytb) gene amplified by the primers L14841 and H15149 (Palumbi et al., Reference Palumbi, Martin, Romano, McMillan, Stice and Grabowski1991) and digested simultaneously by the AluI and the HinfI restriction enzymes and a segment of the cytochrome oxydase I gene (COI) amplified by the primers L5950 and H7196 (Palumbi et al., Reference Palumbi, Martin, Romano, McMillan, Stice and Grabowski1991) and digested by the HhaI restriction enzyme. Both segments, Cytb and COI, were part of the three regions screened by Mamuris et al. (Reference Mamuris, Sfougaris and Stamatis2001). Segment COI was exactly the same and was amplified with the same primers, whereas Cytb is part of the region D-loop/Cytb screened in Mamuris et al. (Reference Mamuris, Sfougaris and Stamatis2001). Double strand DNA amplifications were performed in 100 μl volumes, containing 3 units of Taq polymerase, 1x reaction buffer (500 mM KCl, 200 mM Tris-HCl pH 9.0), 0.2 mM dNTPs, 0.5 mM of each primer, 2 mM MgCl2 and approximately 500 ng of DNA. Polymerase chain reaction (PCR) amplification conditions were as follows: one preliminary denaturation at 95°C for 5 minutes, followed by strand denaturation at 94°C for 1 minute, annealing at 52°C for 30 seconds (Cytb) or 1 minute (COI), and primer extension at 72°C for 30 seconds (Cytb) or 1.5 min (COI)
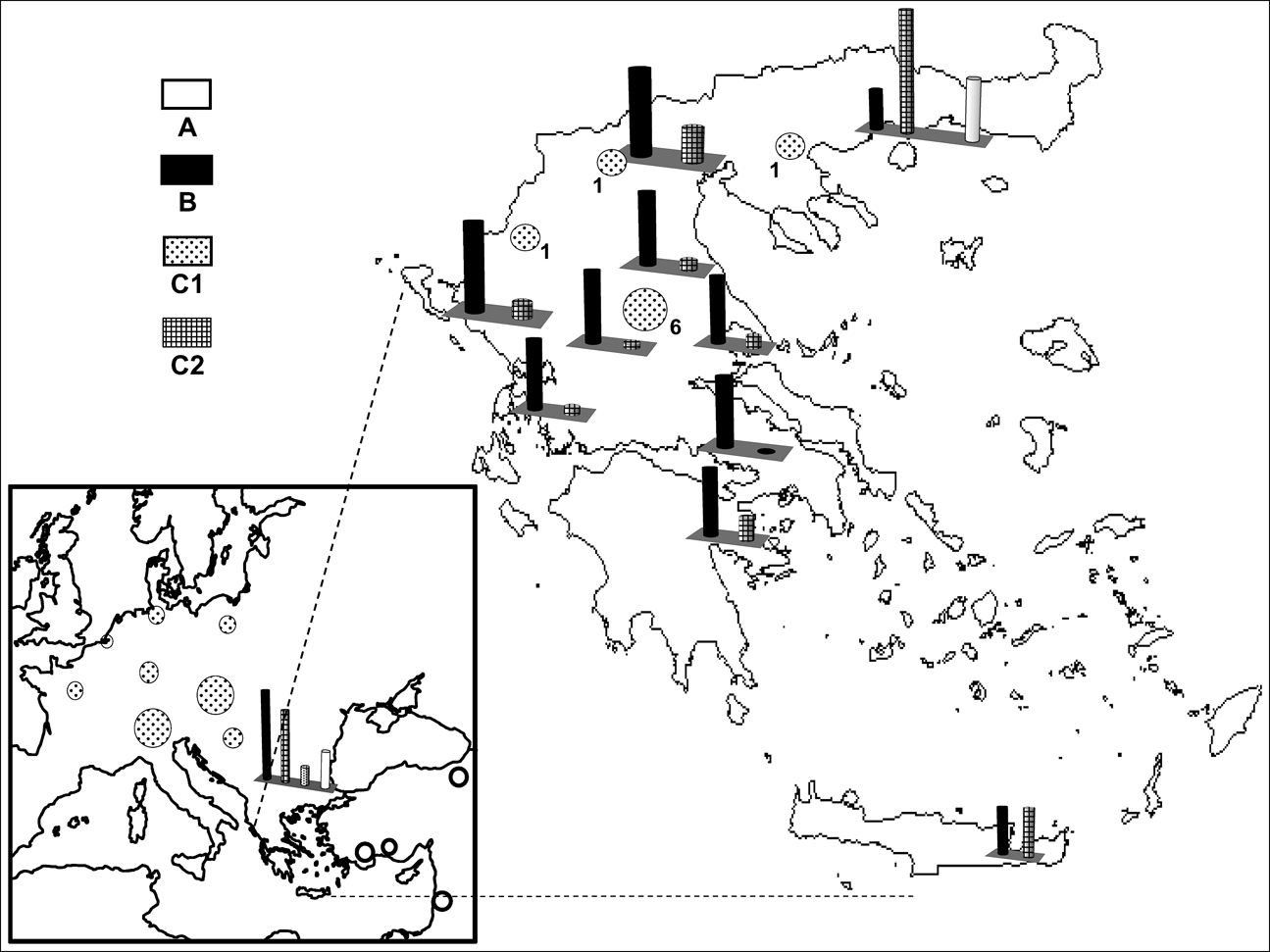
Fig. 1 Sites from which European brown hares were sampled and the occurrence of the mtDNA profiles A, B, C1 and C2 (Fig. 2). Circles and bar charts depict the occurrence of one and multiple haplogroups respectively, at the sampled sites. Numbers correspond to the nine brown hares with profile C1 detected in Greece (see text for details).
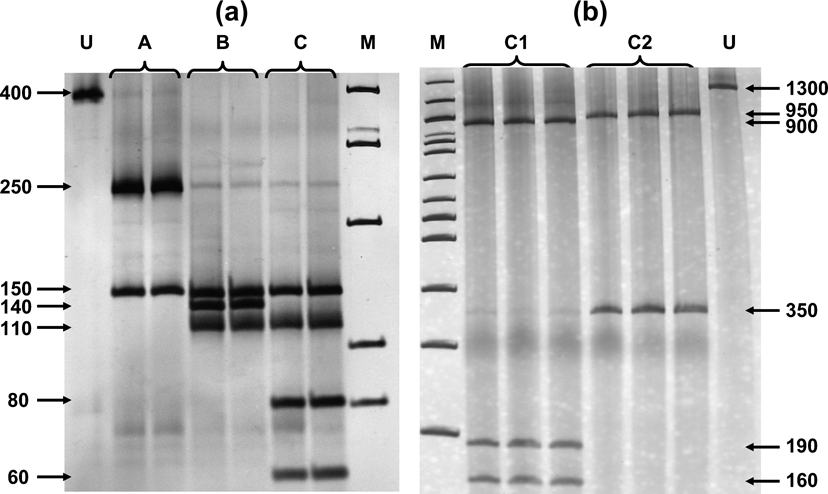
Fig. 2 (a) Digestion of Cytb segment with AluI and HinfI produced three profiles, referred to as A, B and C. (b) Individuals with profile C were further differentiated as C1 and C2 after the digestion of COI segment with HhaI. U = undigested DNA, M = 100bp ladder. Numbers indicate base pairs for each band.
Digestion profiles are shown in Fig. 2, and their geographical occurrence in Fig. 1. The diagnostic enzymes permit the allocation of individuals to the different haplogroups. The results were validated by using both types of analysis (full set of restriction enzymes on the three mtDNA, segments as in Mamuris et al., Reference Mamuris, Sfougaris and Stamatis2001, vs only diagnostic enzymes) in a large proportion of the samples. Profile A was found in all brown hares hunted in Turkey and Israel and a percentage of brown hares from north-eastern continental Greece (15.4%) and Bulgaria (21.8%). Profile B occurred exclusively in Greece and Bulgaria. Hares with the C2 profile apparently occurred in different places both in Greece and Bulgaria but were never traced in Turkey, Israel or in any other European country screened. Analysis of the reared brown hares from the two breeding stations revealed an identical profile (C1) to that traced in all individuals from France, the Netherlands, Germany, Poland, Austria, Switzerland, Serbia and a percentage of hares from Bulgaria. Until 2003, after the analysis of more than 400 individuals (Mamuris et al., Reference Mamuris, Sfougaris and Stamatis2001, and this study), we had never detected the profile C1 in wild brown hares, even in central Greece, which was intensively surveyed. However, monitoring in 2003 and 2004 detected nine brown hares (four females, one male and four of unidentified sex) from central (seven) and northern Greece (two) with the profile C1. Some of those hares previously released in Europe may also have had profile B because hares that were reared in eastern European breeding stations may have been supplied from Bulgaria. However, our genetic data for brown hares from central or north-western Europe did not trace any individual with profile B.
In February 1999 four released brown hares (two males, two females), born in captivity and hard-released, were radio-tracked during a restocking operation (A.I. Sfougaris, pers. comm.). Three died during the first 6 days and the fourth one after 13 days; all four were probably killed by red foxes Vulpes vulpes (A.I. Sfougaris, pers. comm.). Other surveys (Pepin & Cargnelutti, Reference Pepin and Cargnelutti1985; Angelici et al., Reference Angelici, Riga, Boitani and Luiselli1999) also indicate that survival of released captive-bred individuals is low. However, given that restocking operations in Greece were halted in 2001 and that brown hares with profile C1 were not traced in Greece before 2003, the presence of nine individuals with the profile C1 is the first indication that a percentage of the released farm-bred brown hares survived long enough to have had at least one reproductive cycle and transmit their genome.
There are two major management objectives of restocking programmes: to prevent the decline or even the extinction of local populations and/or to increase genetic diversity, reducing the degree of relatedness and inbreeding. Hare densities in central and western Greece, ranging from 1.1 to 2.4 individuals per 100 ha, are much lower than in other European countries (Smith et al., Reference Smith, Vaughan Jennings and Harris2005). Furthermore, during the years 1986-1990, the records of the Ministry of Agriculture show that European brown hare syndrome caused severe mortality (40-90%). In these circumstances, captive breeding and restocking programmes followed by strict genetic control should be considered and evaluated.
In contrast to natural populations, reared populations showed relatively little genetic variation (Mamuris et al., Reference Mamuris, Sfougaris and Stamatis2001, Reference Mamuris, Sfougaris, Stamatis and Suchentrunk2002). From the genetic point of view the only advantage that released animals could confer is the enrichment of the Greek mtDNA genetic pool with the ‘reared’ mtDNA haplotypes. According to our data Bulgaria, and now, after the releases, north-eastern continental Greece, are the only European regions where animals with all four types of mtDNA profile occur. In both cases either there are no data or it is too early to check for possible locally maladapted individuals or genes (Hodder & Bullock, Reference Hodder and Bullock1997).
Studies revealing the richness of the genetic diversity of brown hare populations in Greece in comparison with other European countries (Mamuris et al., Reference Mamuris, Sfougaris and Stamatis2001, Reference Mamuris, Sfougaris, Stamatis and Suchentrunk2002; Suchentrunk et al., Reference Suchentrunk, Mamuris, Sfougaris and Stamatis2003) led to the cessation of releases to protect this genetic diversity. Our data indicate that regional gene pools are differentiating, with a detectable change in the genetic structure of Greek brown hare populations resulting from restocking operations. If, in the long-term, introgressed foreign genes survive, forming new genotypes with indigenous genes, this would demonstrate that they are successful in terms of competition. As nuclear gene pools are not markedly divergent between Greek and other European hares (Mamuris et al., Reference Mamuris, Sfougaris, Stamatis and Suchentrunk2002; Suchentrunk et al., Reference Suchentrunk, Mamuris, Sfougaris and Stamatis2003), foreign nuclear genes should not be a serious handicap. Hence, in certain situations release programmes may be appropriate.
Acknowledgements
We thank the hunting associations for help with sample collection, the staff of the Directorate of Aesthetic Forests, National Parks and Game of the Ministry of Agriculture for providing useful information, and Mrs. A. Voulgari for technical assistance. The Greek Ministry of Agriculture financed part of the sample collection in the course of another project granted to Z. Mamuris.
Biographical sketches
Costas Stamatis is currently working on the phylogeography, population genetics and management of brown hares in Europe. Franz Suchentrunk has a long-term interest in wildlife population genetics and ecology and particularly in mammals, studying, among other things, the evolution of hares. Hakan Sert's main interests are the biodiversity and genetic resources of Anatolian animals, the phylogeography of the Middle East, and ecology and genetics of various animal species in the Middle East. Costas Triantaphyllidis' interests are in animal population genetics, evolution and phylogeography. Zissis Mamuris's research interests include the evolution, phylogeography, population genetics and conservation of animal populations.




