Introduction
Cormorants (Phalacrocoracidae) are piscivorous birds found in coastal areas or inland waters (Cramp, Reference Cramp1977; Johnsgard, Reference Johnsgard1993; Nelson, Reference Nelson2005). They have frequently come into conflict with fishing communities because of apparent competition for fish (Nettleship & Duffy, Reference Nettleship and Duffy1995; Liordos et al., Reference Liordos, Zogaris, Papandopoulos, Van Eerden, van Rijn and Keller2005; Östman et al., Reference Östman, Boström, Bergström, Andersson and Lunneryd2013). Commercial fisheries can experience declines in fish stocks as a result of such competition (Barrett et al., Reference Barrett, Røv, Loen and Montevecchi1990; Suter, Reference Suter1994; Glahn & Brugger, Reference Glahn and Brugger1995; Glahn & Stickley, Reference Glahn and Stickley1995; Price & Nickum, Reference Price and Nickum1995; Weseloh et al., Reference Weseloh, Ewins, Struger, Mineau, Bishop, Postupalsky and Ludwig1995; Östman et al., Reference Östman, Boström, Bergström, Andersson and Lunneryd2013). Cormorants may limit recruitment of declining fish stocks (Barrett et al., Reference Barrett, Røv, Loen and Montevecchi1990); prey on baits used in lobster traps or on fishing hooks, thus reducing capture rates (Price & Nickum, Reference Price and Nickum1995); feed from aquaculture ponds housing a variety of farmed fish (Price & Nickum, Reference Price and Nickum1995); feed on the prey species of commercial fish (Östman et al., Reference Östman, Boström, Bergström, Andersson and Lunneryd2013); and cause significant damage to fishers’ nets while diving in pursuit of fish (Nettleship & Duffy, Reference Nettleship and Duffy1995).
Other studies, however, have demonstrated that cormorants have a positive effect on fisheries and ecosystem dynamics (Suter, Reference Suter1994, Reference Suter1995a,Reference Suterb; Liordos & Goutner, Reference Liordos and Goutner2008). They prey on fish species that are not targeted by humans (e.g. Liordos & Goutner, Reference Liordos and Goutner2008), feed on fish of smaller size classes (Liordos & Goutner, Reference Liordos and Goutner2008; Troynikov et al., Reference Troynikov, Whitten, Gorfine, Pūtys, Jakubavičiūtė, Ložys and Dainys2013), selectively feed on and remove sick fish from farms (Nettleship & Duffy, Reference Nettleship and Duffy1995), and cause density-dependent regulation of fish, thereby enhancing fish diversity and ecosystem functioning (Suter, Reference Suter1994, Reference Suter1995a,Reference Suterb). Fishers generally perceive cormorants to be a threat to fisheries (Stickley & Andrews, Reference Stickley and Andrews1989; Liordos et al., Reference Liordos, Zogaris, Papandopoulos, Van Eerden, van Rijn and Keller2005; Nelson, Reference Nelson2005); for example, Mississippi catfish farmers believed that double-crested cormorants Phalacrocorax auritus were the greatest threat to farmed fish in ponds (Glahn & Brugger, Reference Glahn and Brugger1995), and fishers of the Amvrakikos Gulf, Greece, believed that cormorants were the primary cause of decline in commercial fish stocks (Liordos et al., Reference Liordos, Zogaris, Papandopoulos, Van Eerden, van Rijn and Keller2005). Although some of their perceptions are well founded (Leopold et al., Reference Leopold, van Damme and van der Veer1998), others are baseless (Dalton et al., Reference Dalton, Ellis and Post2009).
The Socotra cormorant Phalacrocorax nigrogularis is a regionally endemic species found in the Arabian Gulf, the Gulf of Oman and adjoining regions (Javed & Khan, Reference Javed and Khan2003; BirdLife International, 2012). It is categorized as Vulnerable on the IUCN Red List, based on significant population declines since the 1980s (BirdLife International, 2012). The cormorants are surface-diving piscivores (Cramp, Reference Cramp1977), and anecdotal information suggests that their diet probably includes sardines Sardinella spp., bigeye Selar crumenophthalmus and yellowtail scads Atule mate, silversides Atherinomorus lacunosus, spotted halfbeaks Hemiramphus far and streaked rabbitfishes Siganus javus (Jennings, Reference Jennings2010). Disturbance at the breeding colonies is regarded as a major threat to the species, and many colonies have gone extinct as a result of oil exploitation and construction (Jennings, Reference Jennings2010). Collection of eggs and chicks occurs in many of the western colonies, including Abu Dhabi (Jennings, Reference Jennings2010; Wilson, Reference Wilson2012), and recreational shooting of adults seems to be a common practice (Jennings, Reference Jennings2010) although this has not been quantified. Egg collection, hunting of chicks or adults, and disturbance to the breeding habitat are all prohibited under United Arab Emirates Federal Law #24 (1999).
The diet of seabirds varies between adults and chicks, and chick-provisioning offers insight into fish availability, distribution patterns and long-term changes in the oceanic environment (Barrett et al., Reference Barrett, Røv, Loen and Montevecchi1990; Montevecchi, Reference Montevecchi, Furness and Greenwood1993). The diet of chicks has therefore been used in many seabird studies to examine feeding and foraging ecology (Schreiber & Burger, Reference Schreiber and Burger2002). Cormorants regurgitate food loads (consisting of intact or partly digested food) and pellets (containing feathers, otoliths or bones) at their nesting sites. Both have been used to determine diet, although pellets provide a higher degree of accuracy (Zijlstra & Van Eerden, Reference Zijlstra and Van Eerden1995) provided libraries of fish otoliths are available. Regurgitated food can also be valuable in identifying dietary components, and is considered to be a suitable index of available diet (Barati, Reference Barati2009; Emmrich & Düttmann, Reference Emmrich and Düttmann2011).
The Arabian Gulf is a shallow, highly saline and relatively warm marine system with rapid turnover; it supports fish communities that are species depauperate compared to other shallow waters at similar latitudes (Sheppard, Reference Sheppard1993; Grandcourt, Reference Grandcourt, Riegl and Purkis2012). Artisanal, recreational and commercial fishing are carried out there, targeting mostly reef fish, including groupers (Serranidae), seabreams (Sparidae), emperors (Lethrinidae), snappers (Lutjanidae), sweetlips and grunts (Haemulidae), jacks (Carangidae) and parrotfishes (Scaridae; Carpenter et al., Reference Carpenter, Krupp, Jones and Zajonz1997; Grandcourt, Reference Grandcourt, Riegl and Purkis2012). Species such as Sardinella longiceps are also exploited as baitfish, although the extent of this exploitation is not reported (MOEW, 2014). In recent years sharks and other elasmobranchs have also been targeted as part of an unregulated market in the United Arab Emirates (Jabado et al., Reference Jabado, Al Ghais, Hamza, Henderson, Spaet, Shivji and Hanner2015). Fisheries statistics are reported at the family level, precluding the estimation of species-specific catch data (MOEW, 2014). The lack of species-specific catch and trade data is regarded as an impediment to adequate fishery management (Jabado et al., Reference Jabado, Al Ghais, Hamza, Henderson, Spaet, Shivji and Hanner2015).
Many fish species are exploited at unsustainable rates in the Arabian Gulf (Grandcourt, Reference Grandcourt, Riegl and Purkis2012; EAD, 2013a; Jabado et al., Reference Jabado, Al Ghais, Hamza, Henderson, Spaet, Shivji and Hanner2015). Populations of the Socotra cormorant could influence fish stocks by targeting bait or commercial fish species. However, a clear understanding of ecological and sociological factors could help to facilitate sustainable coexistence of cormorants (or other seabirds) and humans (Van Eerden et al., Reference Van Eerden, Koffijberg and Platteeuw1995) in the Arabian Gulf. We studied the diet of Socotra cormorant chicks to better understand the species’ role in the Arabian Gulf and its impact on the commercial fishery of the United Arab Emirates.
Study area
Within the Arabian Gulf the largest breeding concentrations of Socotra cormorants occur within the Gulf of Salwa. Bahrain's Hawar Islands complex hosted a population of c. 27,000 pairs during 2005–2006 (Jennings, Reference Jennings2010; Fig. 1), and several Saudi Arabian coastal islands hosted 30,000–40,000 pairs (Jennings, Reference Jennings2010). Colonies in the western United Arab Emirates collectively host c. 12,000 pairs on 3–6 islands in Abu Dhabi (EAD, 2012, 2013a,b), and a single colony on Siniya Island in Umm Al Quwain (Fig. 1) is estimated to host 28,000–35,000 pairs (Muzaffar, Reference Muzaffar and Mahala2014). Although current trends suggest that Siniya Island may have a larger breeding population, we still consider the Hawar Islands to be the largest breeding colony, given the variability in the size of the breeding population between years (Pilcher et al., Reference Pilcher, Phillips, Aspinall, Al-Madany, King and Hellyer2003; Jennings, Reference Jennings2010; Muzaffar, Reference Muzaffar and Mahala2014).
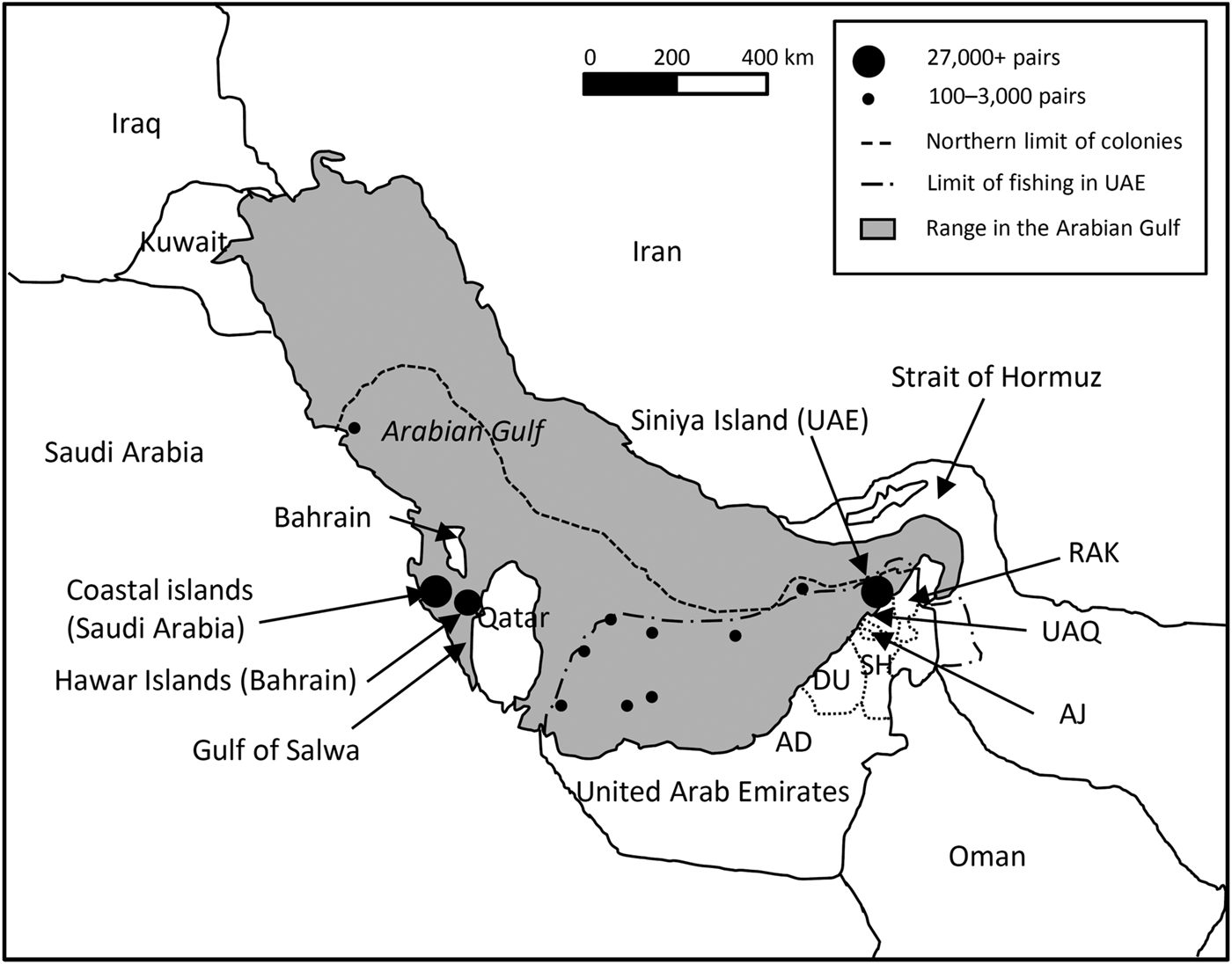
Fig. 1 Distribution of the Socotra cormorant Phalacrocorax nigrogularis in the Arabian Gulf (BirdLife International, 2012). AD, Abu Dhabi; DU, Dubai; SH, Sharjah; AJ, Ajman; UAQ, Umm Al Quwain; RAK, Ras Al Khaimah
This study was part of a greater effort to understand breeding, foraging ecology and conservation of the species on Siniya Island (Fig. 1; see Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012, Reference Muzaffar, Benjamin and Gubiani2013, Reference Muzaffar, Gubiani and Benjamin2015 for details of the site). The colony of Socotra cormorants is restricted to the north-central part of the island. The habitat consists of mixed desert scrub (Haloxylon–Arthrocnemum macrostachyum complex), loose sandy gravel and plantations of Acacia tortilis, Prosopis juliflora and Prosopis cineraria. The island is under the jurisdiction of the Umm Al Quwain Municipality and the Ministry of Environment and Water. The nearest islands with breeding Socotra cormorants are 329 km west, in Abu Dhabi (Jennings, Reference Jennings2010; Fig. 1).
Methods
Fish loads were collected during the 2011–2012 and 2012–2013 breeding seasons. We approached nesting cormorants during 14.00–16.00, which elicited regurgitation (normally they would attempt to feed the chicks directly). In the 2011–2012 breeding season sampling was carried out early (17 October 2011), when most chicks (> 75%) had hatched but were still small and associated with nests, and late (19 November 2011), when chicks were > 2 weeks old and many were still associated with nest sites but most were forming creches (Gubiani et al., Reference Gubiani, Benjamin and Muzaffar2012). Thus, there was a better representation of the diet of chicks of various sizes during 2011–2012. In 2012–2013 fish loads were collected only on two dates during the breeding season (11 November 2012 and 21 February 2013), arbitrarily defined as early and late. Our observations suggested that one species dominated fish loads throughout the season, and therefore the small sample size could still be representative. Each fish load was placed in a labelled ziplock bag and either analysed on site, away from the colony (2011–2012), or frozen within 2–3 hours and processed later in the laboratory (2012–2013). Each species was photographed, and representative specimens were retained to confirm their identification later.
Loads that contained partly digested fish were not used in the analyses. We analysed a total of 27 fish loads containing 330 individual fish in 2011–2012 and 12 fish loads containing 239 individual fish in 2012–2013. Although we recognize that these sample sizes are small and there is potential for bias, we believe that the collection method made sampling representative although it had the potential to miss rare species in the diet.
Each individual fish was identified to the lowest possible taxonomic level using FAO (1974), Carpenter et al. (Reference Carpenter, Krupp, Jones and Zajonz1997) and FishBase (Froese & Pauly, Reference Froese and Pauly2014), and their standard lengths were measured. The biomass of each species was estimated using the equation
where SL = standard length. Values of fitting parameters a and b were obtained from published references for the species or genus in FishBase (Froese & Pauly, Reference Froese and Pauly2014). The total number of fish per load, mean biomass of individual fish from all fish loads, and mean biomass of fish loads were pooled for a given season and compared between years using one-way ANOVA to examine inter-year differences in diet. Relative abundance and fish biomass were also compared between years. Biomass of early and late fish loads was compared using one-way ANOVA.
Breeding population data for the Socotra cormorant were obtained from our ongoing monitoring activity (Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012, Reference Muzaffar, Benjamin and Gubiani2013). The total breeding populations during the 2011–2012 and 2012–2013 seasons were estimated based on the density and area of occupancy of breeding pairs. Area of occupancy was mapped in Google Earth (Google Inc., Mountain View, USA) and calculated in m2. The density of nesting birds was taken from Muzaffar et al. (Reference Muzaffar, Gubiani and Benjamin2012). Variance was estimated following Anderson et al. (Reference Anderson, Henny, Godínez-Reyes, Gress, Palacios and Santos Del Prado2013). Hatching success was estimated to be 58.7% for 2011–2012 (Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012) and 81.2% for 2012–2013 (S.B. Muzaffar, unpubl. data). Fledging success (the proportion of hatchlings that survived to fledging) was estimated to be 65.6 (Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012) and 82.1% (S.B. Muzaffar, unpubl. data) for 2011–2012 and 2012–2013, respectively. Fourteen study plots, each containing 10–30 nests, were monitored once every 2 weeks to determine the hatching success and fledging success in 2011–2012 (Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012). Chicks were considered fledged if they were no longer observed in the nest after 35 days (see details in Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012). Juveniles and non-breeding adults were estimated to comprise 23 and 12%, respectively, of the breeding population each year, based on the age structure of other species of cormorants (Nelson, Reference Nelson2005). Variance in the estimates of the total numbers of chicks and fledglings was based on variation in the hatching and fledging success in 14 plots containing 141 nests in 2011–2012 (Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012) and four plots containing 53 nests in 2012–2013 (S.B. Muzaffar, unpubl. data). Variance in the juvenile, non-breeding adult and breeding adult populations was estimated by resampling the product of the density of nests in the 14 plots (Muzaffar et al., Reference Muzaffar, Gubiani and Benjamin2012) and the area 1,000 times with replacement (Manly, Reference Manly1991).
The number of meals per chick was estimated to be three per day, based on our observations, and it was assumed that one meal represented one fish load. The biomass consumed by the total population of chicks was estimated based on the mean biomass of fish in fish loads consumed during each breeding season (120 days). The biomass consumed by fledged chicks was calculated over the entire year (245 days), assuming none of the fledged chicks died before the following year.
Consumption of fish by juveniles and non-breeding and breeding adults was estimated based on allometric calculations in Nagy (Reference Nagy1987) and then extrapolated to the total population of those age classes in 2011–2012 and 2012–2013. Field metabolic rate (FMR) was calculated using the following equation:
The mass of a Socotra cormorant was set at 1,500 g, based on our measurements of adult individuals (S.B. Muzaffar, unpubl. data). Energy density of prey was set at 5.7 kJ g−1, following Danckwerts et al. (Reference Danckwerts, McQuaid, Jaeger, McGregor, Dwight, Le Corre and Jaquemet2014). All quantities of fish consumed were converted to tonnes and extrapolated to the entire population in each year.
Fishing occurs extensively within the waters of the United Arab Emirates (Fig. 1) and some areas are fished by more than one emirate. Fisheries statistics for 2011 were obtained from the Ministry of Environment and Water (MOEW, 2014). Fisheries statistics for 2012 were not available and were estimated based on the mean incremental increase in fish landings during 2008–2011 available from the MOEW. These data provide total fish catches in all seven emirates (Abu Dhabi, Dubai, Sharjah, Ajman, Umm Al Quwain, Ras Al Khaimah and Fujairah), including total catch, total catch of demersal fish and total catch of pelagic fish. Based on foraging data for Socotra cormorants (Muzaffar, Reference Muzaffar and Mahala2014) we determined that individuals breeding on Siniya Island were fishing in the waters of Dubai, Sharjah, Ajman, Umm Al Quwain and Ras Al Khaimah. We therefore pooled the landing data for these five emirates to compare total biomass consumption by Socotra cormorants in relation to fish landings. The accuracy of the data for fish catches is not indicated on the MOEW website, and thus we have presented them as raw total catch, without any standard error. All statistical analyses were conducted in Minitab 14.1 (Informer Technologies Inc., Shingle Springs, USA).
Results
Fish loads contained 1–66 fish, with a mean of 12.2 in 2011–2012 and 19.2 in 2012–2013; the difference was not significant (Fig. 2). Mean biomass of individual fish was significantly higher in 2011–2012 (4.58 g) compared to 2012–2013 (2.53 g; one-way ANOVA, n = 569, F = 14.97, P < 0.001). Total biomass per fish load, however, was not significantly different between years.
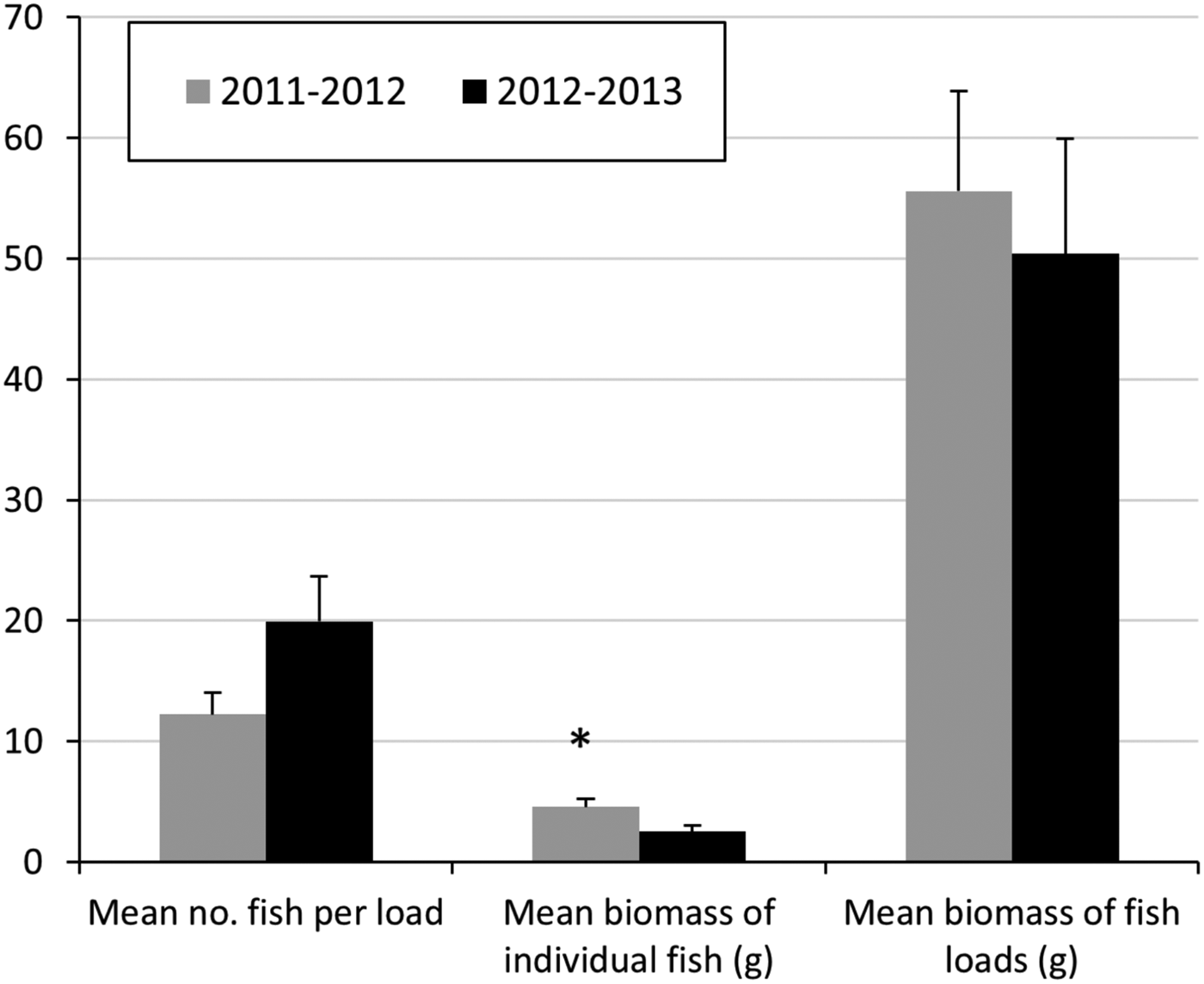
Fig. 2 Comparison of fish consumption by Socotra cormorants on Siniya Island (Fig. 1) during the 2011–2012 and 2012–2013 breeding seasons. The asterisk indicates significant difference at α = 0.05.
Seven species of fish were recorded in the fish loads: sailfin flying fish Parexocoetus mento, blue-stripe sardine Herklotsichthys quadrimaculatus, anchovies Encrasicholina spp., pink ear emperor Lethrinus lentjan, pickhandle barracuda Sphyraena jello, congaturi halfbeak Hyporhamphus limbatus, and bigeye scad Selar crumenophthalmus (Fig. 3). There was little overlap in the composition of the diet between the 2 years. Sailfin flying fish were the most numerically dominant species in 2011–2012 (70%), whereas anchovies dominated in 2012–2013 (98.32%). Estimates of fish biomass revealed that the blue-stripe sardine was more prevalent as prey in 2011–2012 (41.47%), followed by the sailfin flying fish (27.96%). In 2011–2012 the mean biomass of fish loads increased from 2.00 to 11.13 g during the chick-rearing period (one-way ANOVA, F = 117.45, P < 0.001). The diet was composed primarily of sailfin flying fish (99.01%) in the early part of the season, followed by a switch to blue-stripe sardine (60.75%) and pink ear emperor (39.25%). No such change was evident in the 2012–2013 season, and anchovies remained the most consumed species throughout the season (100% early in the season and 94.1% later). Biomass of fish per load decreased between early (3.53 g) and later (1.55 g) in the season (one-way ANOVA, F = 374.89, P < 0.001).

Fig. 3 Comparison of the diet of Socotra cormorants on Siniya Island (Fig. 1) during the 2011–2012 and 2012–2013 breeding seasons in terms of (a) number of individuals and (b) biomass of prey species.
Total consumption of fish was estimated to be 11,626 ± SE 72 t in 2011–2012 and 18,764 ± SE 126 t in 2012–2013. Chicks consumed c. 808 ± SE 32 and 1,491 ± SE 51 t during the chick-rearing period in 2011–2012 and 2012–2013, respectively (Table 1). Fledglings consumed 2,344 ± SE 93 and 5,960 ± SE 206 t in 2011–2012 and 2012–2013, respectively. Total fish biomass consumed by the Socotra cormorant population of Siniya Island was 11,626 ± SE 72 and 18,764 ± SE 126 t in 2011–2012 and 2012–2013, respectively. The total tonnage of fish landed by the commercial fisheries from the foraging areas of Socotra cormorants was 56,557 t in 2012 and 62,972 t in 2013. Most of the catch was composed of demersal fishes (32,781 t in 2012, 36,500 t in 2013) and none of the commercial catches included any of the species that were dominant in the diet of Socotra cormorants (MOEW, 2014; Table 2).
Table 1 Population size of Socotra cormorants Phalacrocorax nigrogularis on Siniya Island, Umm Al Quwain, United Arab Emirates (Fig. 1), and the estimated biomass consumed by the population during the 2011–2012 and 2012–2013 breeding seasons.
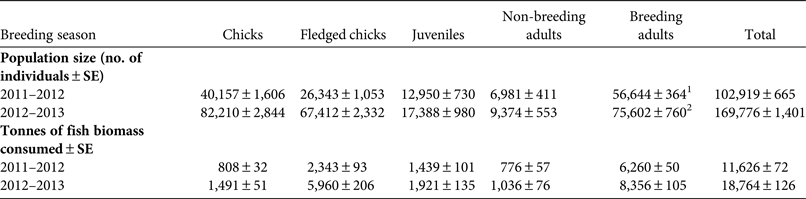
1 28,322 breeding pairs
2 37,801 breeding pairs
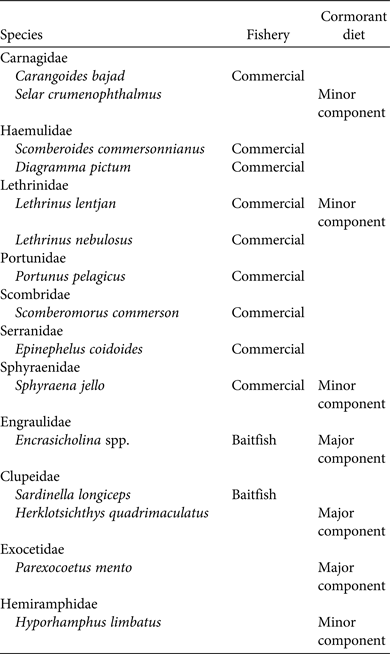
Table 2 Overlap between major fish species targeted commercially or as baitfish and the diet of Socotra cormorants on Siniya Island, United Arab Emirates (Fig. 1).
Discussion
Cormorant–fisheries interactions remain a contentious issue in wildlife management. Increases in populations of great cormorants Phalacrocorax carbo are usually met with aggressive management intervention, primarily to protect fisheries (Vetemaa et al., Reference Vetemaa, Eschbaum, Albert, Saks, Verliin and Jürgens2010; Emmrich & Düttman, Reference Emmrich and Düttmann2011). Fine-scale studies have helped to refute or confirm the suspected negative impact of cormorants on fisheries (Van Eerden et al., Reference Van Eerden, Koffijberg and Platteeuw1995; Vetemaa et al., Reference Vetemaa, Eschbaum, Albert, Saks, Verliin and Jürgens2010; Emmrich & Düttman, Reference Emmrich and Düttmann2011). The Arabian Gulf has a relatively small volume and is semi-isolated, with a species-poor fish fauna derived from the adjacent Arabian Sea (Sheppard, Reference Sheppard1993; Grandcourt, Reference Grandcourt, Riegl and Purkis2012). Commercial fishing in the Arabian Gulf has increased in recent years as a result of development in the Middle East (Grandcourt, Reference Grandcourt, Riegl and Purkis2012; Jabado, Reference Jabado2014), and is dominated by medium–large reef fish species belonging to the Serranidae, Sparidae, Lethrinidae, Lutjanidae and Carangidae (Carpenter et al., Reference Carpenter, Krupp, Jones and Zajonz1997; EAD, 2012; Grandcourt, Reference Grandcourt, Riegl and Purkis2012).
The breeding population of Socotra cormorants of Siniya Island may have a significant impact on its food webs, utilizing a variety of small fish of length 45–180 mm. The diet of chicks varied significantly between years, suggesting that the species is a generalist, feeding opportunistically on abundant fish species. The local fisheries primarily land large demersal or reef fishes, whereas the diet of cormorant chicks consists of small, pelagic fishes, mostly of low commercial value, and therefore the impact of the cormorants on fisheries is minimal.
Chick diet
Socotra cormorants appear to be a generalist species, with significant variation in the diet of chicks. The diet consisted mostly of sailfin flying fish and blue-stripe sardines in 2011–2012 and anchovies in 2012–2013. The fishes consumed were small (biomass 2–6 g), with significantly lower individual biomass recorded in 2012–2013, when anchovies comprised 98% of the diet. The mean biomass of fish loads was not significantly different between years, however, indicating that parents provided approximately the same amount of food to their growing chicks, compensating for differences in the biomass of individual fish species. As partly digested fish were not included in the analyses, rare species were probably not adequately sampled and only dominant species were quantified, and therefore further studies are needed to characterize the breadth of the diet of cormorants.
Many cormorant species exhibit opportunistic dietary patterns over the course of a year (Nelson, Reference Nelson2005; Liordos & Goutner, Reference Liordos and Goutner2008), suggesting adaptability to changing fish stocks. Jennings (Reference Jennings2010) reported that sardines, halfbeaks, scads and rabbitfish formed part of the diet of Socotra cormorants in the Hawar Islands in Bahrain. Although these species were not recorded in our study, some are from the same families (pelagic species of Hemiramphidae, Clupeidae and Carangidae). This indicates that cormorants from different colonies may have dissimilar diets, depending on the available local fish resources, seasonality and year, although the major fish groups targeted may be similar. Intercolony variation in diet has been widely reported in other cormorant (Liordos & Goutner, Reference Liordos and Goutner2008) and seabird species (Schreiber & Burger, Reference Schreiber and Burger2002) and requires further investigation in Socotra cormorants.
Mean biomass of fish load increased significantly during the latter part of the 2011–2012 season. This was probably a reflection of the breeding phase, with larger chicks compelling parents to provide larger fish loads. The change in fish biomass also reflected a switch from mainly flying fish to sardines, as parents provided more nutritious food to their growing chicks (Barati, Reference Barati2009; Emmrich & Düttmann, Reference Emmrich and Düttmann2011). The diets of great cormorants are also known to change in response to the nutritional requirements of growing chicks during the course of a season (Liordos & Goutner, Reference Liordos and Goutner2008; Barati, Reference Barati2009; Emmrich & Düttmann, Reference Emmrich and Düttmann2011).
In contrast, in 2012–2013 the biomass of fish loads declined as the season progressed. A larger sample size could have provided a better understanding of how fish loads change in relation to fish availability; however, we speculate that difference in the timing of breeding between 2011–2012 and 2012–2013 could explain our observations. In 2012–2013 breeding started c. 2 weeks later and peak breeding was observed in December, compared to November in 2011–2012. Anchovies in the Arabian Gulf migrate westwards from the northern emirates (including Umm Al Quwain) in December and become less abundant after January (MOEW, pers. comm.). Thus, a more prolonged breeding season in 2012–2013 could have caused a decline in the local stocks of anchovies, which was reflected in the lower biomass of fish loads observed late in the season. A more detailed investigation of seasonal changes in diet is necessary to better understand dietary shifts in breeding colonies of Socotra cormorants.
Impact on fisheries
Great cormorants have contrasting impacts on local fish resources. Increasing populations caused a decline in fish stocks in an estuary in Estonia over a 10-year period (Vetemaa et al., Reference Vetemaa, Eschbaum, Albert, Saks, Verliin and Jürgens2010). However, Engström (Reference Engström2001) showed that although great cormorant populations were linked with fish densities, predation pressure from cormorants did not cause a decline in fish densities in Lake Ymsen, Sweden. Thus, the perceived impact of cormorants on fisheries is often incorrect. Troynikov et al. (Reference Troynikov, Whitten, Gorfine, Pūtys, Jakubavičiūtė, Ložys and Dainys2013) showed that great cormorants selected fish of size classes that did not overlap with the fish targeted by the European perch Perca fluviatilis fishery in Lithuania. Furthermore, great cormorants may select different species from those targeted by fisheries in Greece (e.g. Liordos & Goutner, Reference Liordos and Goutner2008). The fish landings of the United Arab Emirates have been increasing steadily since the 1970s (Beech et al., Reference Beech, Al Abdussalam, Hoolihan, Hellyer and Aspinall2005; Grandcourt, Reference Grandcourt, Riegl and Purkis2012). The fisheries statistics reports for the emirate of Abu Dhabi suggests that the bulk of the fish catch comprises orange-spotted trevally Carangoides bajad, Talang queenfish Scomberoides commersonnianus, painted sweetlips Diagramma pictum, pink ear emperor, spangled emperor Lethrinus nebulosus, blue crab Portunus pelagicus, narrow-barred Spanish mackerel Scomberomorus commerson, orange-spotted grouper Epinephelus coioides and pickhandle barracuda (collectively > 3,960 t per year, 83% of total catch in Abu Dhabi in 2012; EAD, 2013b). Similar data for other emirates are not available but it may be assumed that they exploit similar species, given the widespread popularity of these species (Beech et al., Reference Beech, Al Abdussalam, Hoolihan, Hellyer and Aspinall2005; Grandcourt, Reference Grandcourt, Riegl and Purkis2012). The sailfin flying fish, which comprised 27.9% of the diet of Socotra cormorants in 2011–2012, is not exploited by the fishery, and the blue-stripe sardine, which dominated the diet (41.5%), is exploited for consumption, baitfish or animal feed but is not reported in the fisheries statistics (Grandcourt, Reference Grandcourt, Riegl and Purkis2012; EAD, 2013a; MOEW, 2014). Similarly, anchovies, which dominated the Socotra cormorant's diet in 2012–2013 (95.9%), are used as baitfish or animal feed but their exploitation is not of commercial importance. It is possible that Socotra cormorants may influence commercial fish species indirectly by eating younger individuals or the prey of the larger fish. Our study does not provide evidence of significant consumption of any larval stages of commercially important fish. The two commercially exploited species found in the diet, S. jello and L. lenjtans, constituted minor components of the diet. Selar crumenophthalmus is not reported in the fisheries statistics, although other species of scads (e.g. Atule bajad) are targeted at low levels (< 60 t annually; EAD, 2012) by the commercial fishery. Anchovies were the most important component of the diet in the 2012–2013 breeding season, suggesting that there could be conflict between cormorants and fishers. However, species that are considered baitfish (e.g. Sardinella longiceps, Encrasicholina spp.) seem to be captured opportunistically and at sufficiently low levels that they are not reported in the fishery statistics (EAD, 2013a; MOEW, 2014). Reports of fishers' attitudes towards Socotra cormorants are anecdotal, and formal surveys are needed to determine perceptions and attitudes of fishers.
The total quantity of fish consumed by the Socotra cormorant population breeding on Siniya Island is small compared to the total tonnage of exploited species (MOEW, 2014; Table 1). Juveniles and breeding and non-breeding adults leave the colony to disperse as far as Abu Dhabi in the west and across the Strait of Hormutz into the Gulf of Oman in the east (Muzaffar, Reference Muzaffar and Mahala2014), thus they forage away from the breeding colony during April–September, exploiting other regions of the Gulf. Based on the little overlap between the diet of cormorant chicks and commercially exploited species, and the relatively small biomass consumed by cormorants, we suggest that the species has a low impact on the commercial fisheries of the United Arab Emirates. The negative perception among fishers of the effects of cormorants on fish stocks in the United Arab Emirates or the greater Arabian Gulf is misplaced.
Measures must be taken to ensure sustainability of fisheries and to protect the Socotra cormorant, especially on Siniya Island. As the United Arab Emirates hosts 41% of the global population of the species, protection and better understanding of Siniya Island and other colonies in Abu Dhabi are essential. Siniya Island hosts the largest population of breeding Socotra cormorants in the United Arab Emirates (Jennings, Reference Jennings2010). Long-term studies examining diet of this and other seabird species will help to understand the role of Socotra cormorants and other seabirds in shaping the food webs of the Arabian Gulf.
Acknowledgements
We thank the Ministry of Environment and Water for providing funding to SBM and logistical support for this work. Additional support for the project was received through the Mohammad Bin Zayed Species Conservation Fund and the United Arab Emirates University/National Research Foundation grant to SBM. We thank the Umm Al Quwain Municipality for issuing permits to work on the island. All field procedures adhere to international standards that were approved by the Animal Research Ethics Committee (Protocol No. A18-12) of UAE University, the Ministry of Environment and Water, and the Umm Al Quwain Municipality.
Biographical sketches
Sabir Bin Muzaffar's research focuses on the movement ecology, breeding biology and conservation of birds. Robert Gubiani works on a variety of environmental projects relating to biodiversity and conservation. Sonya Benjamin's research interests focus on determining the effectiveness of common ecological mitigation measures often proposed as a means of alleviating adverse impacts from developmental pressures in the Middle East. Rashid AlShihi's research interests focus on marine organisms, including sea birds, and marine ecosystems and conservation. Ahmed Al-Romithi has worked on the diet and foraging of cormorants. Faisal Al Kaabi is interested in the feeding ecology of cormorants.







