Introduction
There is an extensive literature on the effects of roads on wildlife and environments (e.g. Forman, Reference Forman, Sperling, Bissonette, Clevenger, Cutshall and Dale2003; van der Ree et al., Reference van der Ree, Smith and Grilo2015). However, many road ecology studies have focused on terrestrial species in temperate environments, especially in areas with highly developed road infrastructure, such as Europe and North America (Holderegger & Di Giulio, Reference Holderegger and Di Giulio2010). These studies have focused on large, paved freeways and finding practical solutions to the problems posed by roads (Smith et al., Reference Smith, van der Ree, Rosell, van der Ree, Smith and Grilo2015). Arboreal animals face additional challenges, as they must descend to the ground to cross roads (Soanes & van der Ree, Reference Soanes, van der Ree, van der Ree, Smith and Grilo2015). However, connectivity of habitat across roads, both natural and anthropogenic, can mitigate the negative effects of roads if arboreal animals traverse these connections (Van der Hoeven et al., Reference Van der Hoeven, de Boer and Prins2010). There are relatively fewer data on how arboreal animals respond to roads in tropical forest regions, particularly for species found in rare tropical dry forests, which are amongst the world's most threatened ecosystems (Sagar & Singh, Reference Sagar and Singh2006). Thus, hypothesis-driven studies are needed in the tropics to determine the impact of roads, rather than an a priori assumption of roads as barriers or vice versa.
Road ecology in Madagascar is particularly important because 94% of lemur taxa are currently listed by IUCN as threatened with extinction (Schwitzer et al., Reference Schwitzer, Mittermeier, Johnson, Donati, Irwin and Peacock2014) and expanding road construction may be a major cause of lemur habitat loss (Rogers et al., Reference Rogers, Glew, Honzák and Hudson2010), although there has been little empirical testing of this assumption. The lack of data is surprising given that roads bisect or border some of the largest and best-known protected areas on the island, such as Ranomafana and Ankarafantsika National Parks.
We investigated whether individual golden-brown mouse lemurs Microcebus ravelobensis were able to cross roads, and how their movement was affected in road environments with various levels of canopy connectivity and uninterrupted forest habitat along a national highway in Ankarafantsika National Park. We predicted that (1) individual lemurs would be able to cross roads when canopy connectivity was present, (2) individual lemurs in uninterrupted forests would not be hindered in their ability to move, and (3) there would be no significant effect of sex on the ability of lemurs to cross roads, as both males and females of the species disperse. These predictions were evaluated to test the hypothesis that roads were a barrier to movement in M. ravelobensis.
Methods
We conducted a capture–mark–recapture study during the dry season (May–August) of 2015 in the Ampijoroa Forest Reserve within Ankarafantsika National Park. There are various microhabitats within the Park (Rakotondravony & Radespiel, Reference Rakotondravony and Radespiel2009), and Ampijoroa is characterized by infertile sandy soils with low water-holding capacities, gallery forest alongside Lake Ravelobe, and a high occurrence of non-native plantation trees, such as Mangifera indica and Tectona grandis (Rendigs et al., Reference Rendigs, Radespiel, Wrogemann and Zimmermann2003; Crowley et al., Reference Crowley, McGoogan and Lehman2012). Route Nationale 4 (RN4) is a 580 km two-lane paved highway from Antananarivo to Mahajanga, which runs directly through Ankarafantsika National Park in a north–south direction for c. 20 km (Fig. 1), dividing the Park into two sections, of 17,500 and 78,000 ha (Radespiel et al., Reference Radespiel, Rakotondravony and Chikhi2008).
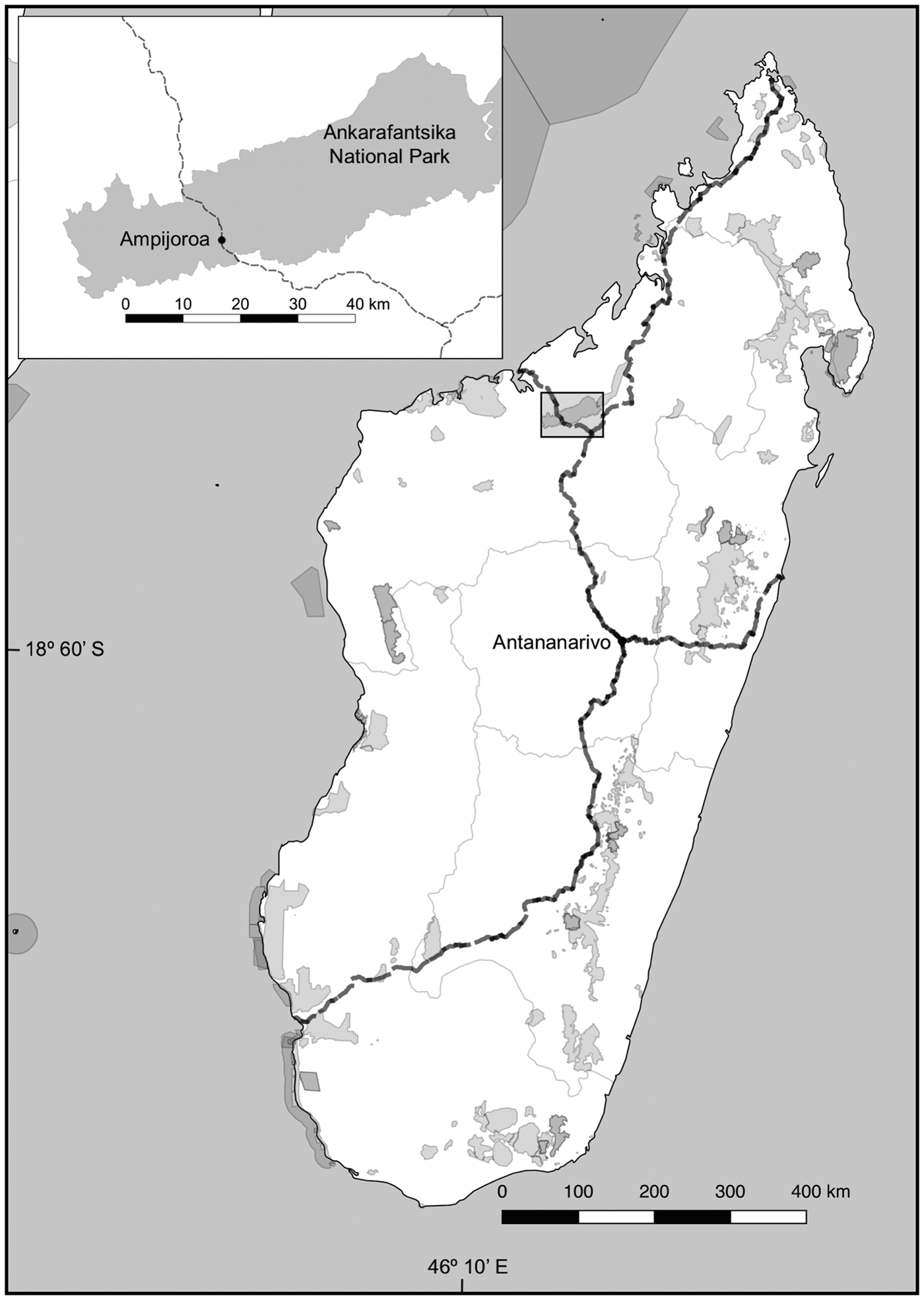
Fig. 1 Major national highways (dashed lines) and protected areas (grey shading) in Madagascar. The inset shows the division of Ankarafantsika National Park by Route Nationale 4 (RN4) into areas of c. 17,500 and 78,000 ha.
Mouse lemurs are small-bodied (30–70 g), nocturnal and solitary omnivores (Kappeler & Rasoloarison, Reference Kappeler, Rasoloarison, Goodman and Benstead2003). Individuals of our study species, M. ravelobensis, establish mixed-sex sleeping sites, their diets contain a higher percentage of arthropods, and they have a slightly sex-biased system of female philopatry, but both sexes disperse (Radespiel et al., Reference Radespiel, Ehresmann and Zimmermann2003, Reference Radespiel, Rakotondravony and Chikhi2008; Weidt et al., Reference Weidt, Hagenah, Randrianambinina, Radespiel and Zimmermann2004). Previous studies have suggested M. ravelobensis populations may be affected by roads, but because the road construction was relatively recent, no explicit genetic barrier was found (Radespiel et al., Reference Radespiel, Rakotondravony and Chikhi2008). Mouse lemurs are excellent model organisms to distinguish between the direct and secondary effects of roads because they are under less direct hunting pressure than larger-bodied animals (Garcia & Goodman, Reference Garcia and Goodman2003).
We established six parallel trap lines of 475 m length at three locations in Ampijoroa (Fig. 1): two on either side of RN4 where there was canopy connectivity across the highway (Road 1), two on either side of RN4 at a site with no canopy connectivity (Road 2), and two within the interior forest, c. 1 km from the road or forest edge, as a control (Forest Control). We sampled trap lines on a 6-day randomized schedule; no trap line was sampled on 2 consecutive nights and monthly trapping intensity was equal at all sites.
We set live animal traps (Sherman, Tallahassee, USA) at 25 m intervals along each trap line, giving a total of 20 trapping sites per trap line and 40 traps at each location. We established the parallel interior transects at similar distances to those on the roads, c. 25 m apart. Upon capture, individuals were categorized by sex and age-class and were marked subcutaneously with a Trovan microchip. For a more detailed description of trapping procedures see Burke & Lehman (Reference Burke and Lehman2014). Our study was approved by the University of Toronto Office of Research Ethics (Protocol #20011050) and complied with all legal requirements of Madagascar.
A crossing event was defined as when an individual was captured on one trap line and then recaptured on the parallel trap line. We used t-tests, χ2 tests, a one-way ANOVA, and Tukey's post-hoc analysis with α = 0.05 to test for differences in movement and capture rates between locations and sex categories. Movement was quantified by rounding distances between adjacent non-diagonal traps to 25 m. All tests were performed in R v. 3.2.2 (R Development Core Team, 2013).
Results
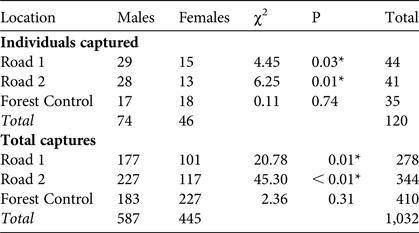
We captured 120 M. ravelobensis individuals in 1,032 capture events at the three locations over 2,245 trap nights (2,520 traps set, with 82 rodent captures and 144 trap malfunctions). There were no significant differences between trapping locations in terms of rates of rodent captures (t = 0.34, df = 60, P = 0.71) or trap malfunctions (t = 1.99, df = 60, P = 0.14). There were significantly more males caught at Road 1 and Road 2 than at Forest Control; males were also caught at a higher rate than females at Road 1 and Road 2, whereas at Forest Control there were no significant differences in the number of individuals caught by sex (Table 1).
Table 1 Number of individuals captured (individuals identified and given a unique ID) and total number of captures (including recaptures) of golden-brown mouse lemurs Microcebus ravelobensis at the Road 1 (connection across RN4), Road 2 (no connection across RN4) and Forest Control (interior forest with no barrier) sites in the Ampijoroa Forest Reserve within Ankarafantsika National Park, Madagascar (Fig. 1), with χ2 and P (* denotes significance at P < 0.05).
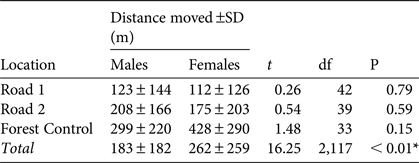
We found no significant differences in mean distance travelled by males and females at any of the three trapping locations (Fig. 1, Table 2). There was a significant difference in total movement between the three locations (Table 2). Tukey's post hoc analysis indicated there was a significant difference between Forest Control and Road 1 (Q = 5.68, df = 2,117, P < 0.001) and Road 2 (Q = 7.78, df = 2,117, P < 0.01), but no significant difference between Roads 1 and 2 (Q = 2.21, df = 2,117, P > 0.05).
Table 2 Distance moved (± SD) by male and female individuals of M. ravelobensis at the Road 1 (connection across RN4), Road 2 (no connection across RN4) and Forest Control (interior forest with no barrier) sites in the Ampijoroa Forest Reserve (Fig. 1), with results of t-tests and ANOVA (* denotes significance P < 0.05).
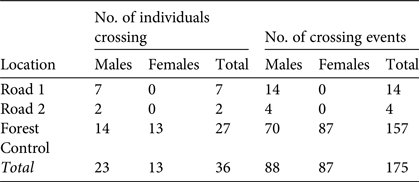
We detected road crossing on 18 occasions, by 9 males (Table 3). Of these 18 captures, 14 (seven individuals) were at Road 1. At Road 2 we captured two individuals four times. Individuals (n = 5) at both locations were recaptured on their original trap line after being caught on the parallel line. At Forest Control we recaptured M. ravelobensis 157 times on a different trap line than that on which they were previously caught, corresponding to 77% (n = 27 of 35) of individuals.
Table 3 The number of individuals of M. ravelobensis captured on both parallel trap lines (individuals crossing), and the total number of captures in which the previous capture was on the parallel trap line (crossing events), at the Road 1 (connection across RN4), Road 2 (no connection across RN4) and Forest Control (interior forest with no barrier) sites in the Ampijoroa Forest Reserve (Fig. 1).
Discussion
Our study is the first to directly test the permeability of roads to mammal movement in Madagascar, and our findings indicate that RN4 affects the movement of M. ravelobensis in Ankarafantsika National Park. Our first two predictions were validated: although the lemurs were able to cross RN4, they did so at a lower rate than if there had been undisturbed canopy. Thus, it is important for conservation managers to protect existing crossings and encourage more canopy growth, as our results suggest that crossing movement of M. ravelobensis would be further reduced if existing arboreal bridges were lost. However, Madagascar is prone to extreme climatic events, such as cyclones, droughts and wildfires (Ingram & Dawson, Reference Ingram and Dawson2005), and in 2000 Cyclone Hudah resulted in the destruction of 51% of trees and an 83% reduction in mean tree crown volume in the Masoala Peninsula (Birkinshaw & Randrianjanahary, Reference Birkinshaw and Randrianjanahary2007). Cyclones of a similar nature and intensity occur in Ankarafantsika National Park (Aymoz et al., Reference Aymoz, Randrianjafy, Randrianjafy and Khasa2013). Additionally, if construction occurs along RN4, anthropogenic damage to these natural arboreal bridges may also occur (Weller, Reference Weller, van der Ree, Smith and Grilo2015). Thus, it would also be prudent for managers to investigate the efficacy of permanent anthropogenic crossing structures, such as bridges or culverts.
Our third prediction was not supported: only male M. ravelobensis were observed crossing RN4 and there were significantly more males alongside RN4 than within the interior forest. Although we captured significantly more males than females at Roads 1 and 2, we captured 15 and 13 individual females, respectively, over 100 times at each location. Additionally, at Forest Control we observed an equivalent number of crossings by males and females (13 and 14, respectively), and thus we consider our results to be robust. A sex-biased effect of roads has also been observed in North American freshwater turtles (Order Testudines), with female turtles more likely to be killed by vehicles than males while crossing roads to find nesting sites, leading to skewed sex ratios (Steen et al., Reference Steen, Aresco, Beilke, Compton, Condon and Dodd2006). However, our results indicate that male M. ravelobensis are more likely to face mortality, as the crossing sex. Given that male and female M. ravelobensis have home ranges of equivalent size and both sexes disperse, this effect must be attributable to other factors, such as competition between males (Weidt et al., Reference Weidt, Hagenah, Randrianambinina, Radespiel and Zimmermann2004) or home range pile-up, in which the lemurs are not affected by roads as direct barriers to dispersal, but through many individual or group home ranges abutting roads (Riley et al., Reference Riley, Pollinger, Sauvajot, York, Bromley, Fuller and Wayne2006). This phenomenon can have significant genetic and social consequences in gregarious species such as mouse lemurs.
Our results on the roadside movement of M. ravelobensis are some of the first such data available for mammals in Madagascar, and contribute valuable data on the effects of roads on the understudied guild of arboreal mammals, for which research is especially needed in tropical environments such as Madagascar (Lehman et al., Reference Lehman, Radespiel, Zimmermann, Lehman, Radespiel and Zimmermann2016). Managers of protected areas such as Ankarafantsika that are intersected or abutted by roads should investigate the efficacy of mitigation measures such as bridges or culverts (Smith et al., Reference Smith, van der Ree, Rosell, van der Ree, Smith and Grilo2015) in aiding the movement of wildlife. However, anthropogenic bridges are potentially costly for developing nations, and have shown mixed results in aiding movement across barriers in Madagascar specifically (Mass et al., Reference Mass, Rakotomanga, Rakotondratsimba, Razafindramisa, Andrianaivomahefa and Dickinson2011). Our results suggest that natural bridges can aid movement, but further study comparing the efficacy of natural and anthropogenic bridges along RN4 and other roads in Madagascar is needed to clarify which is more effective. Given the urgent conservation needs and the rapid development of roads within Madagascar, more hypothesis-driven studies are needed on the effects of roads, to inform conservation initiatives.
Acknowledgements
We thank the staff of Madagascar Institute pour la Conservation des Environnements Tropicaux, Madagascar National Parks and La Maison du Pyla. We also thank Becky Raboy, Ute Radespiel, Elke Zimmerman, Jhonny Kenedy, Hajanirina Ravelonjanahary, Annette Klein, Mamy Rina Evasoa and Alida Hasiniaina for theoretical and practical assistance, and Amy Mui and two anonymous reviewers for valuable comments on this article. Funding for this study was provided by Primate Conservation Inc., Sigma Xi Grants-in-Aid of Research, University of Toronto Department of Anthropology, and Natural Science and Engineering Research Council of Canada grants to SML (Discovery Grant) and MSR (CGS-M).
Author contributions
MSR conducted all data analysis and drafted the article. MSR and AR collected the data, assisted by SML. AR and SML assisted in designing the study and preparing the article.
Biographical sketches
Malcolm Ramsay’s research focuses on lemur adaptations to complex anthropogenic environments. He is also interested in outcomes of applied conservation biology research in Madagascar and elsewhere. Andriamahery Razafindrakoto works on Microcebus as a model organism for primate biology, behaviour and health. Shawn Lehman’s long-term research objective is to integrate evolutionary ecology and conservation biogeography to model responses of primates to anthropogenic disturbances.






