Introduction
The proportion of males and females, i.e. sex ratio, is an important factor of success for a newly founded population (Sarrazin & Legendre, Reference Sarrazin and Legendre2000). According to Fisher's (Reference Fisher1930) and Trivers’ (Reference Trivers and Campbell1972) theories on sexual selection, equilibrated sex ratio at birth is expected in monogamous species (Cockburn et al., Reference Cockburn, Legge, Double and Hardy2002) but differential pressures on sexes could occur and lead to skewed mortality or dispersal (Gowaty, Reference Gowaty1993; Bradshaw et al., Reference Bradshaw, Harcourt and Lloyd2003). Sex ratio among sexually mature individuals is often male biased for monogamous species (Bessa-Gomes et al., Reference Bessa-Gomes, Legendre and Clobert2004). Moreover, for monogamous species, stochastic fluctuations of sex ratio in a small population could lead to higher extinction probability than in other mating systems (Legendre et al., Reference Legendre, Clobert, Møller and Sorci1999) and could affect effective population size by increasing reproductive variance among individuals (Anthony & Blumstein, Reference Anthony and Blumstein2000). Biased sex-ratio may also cause an Allee effect, mostly because of limited mate finding (Stephens & Sutherland, Reference Stephens, Sutherland, Apollonio, Festa-Bianchet and Mainardi2000; Engen et al., Reference Engen, Lande and Sæther2003).
Therefore, the optimal sex ratio among sexually mature individuals required to maximize reintroduction success with monogamous species is 1:1 because this ratio will maximize population growth rate (Legendre, Reference Legendre, Ferrière, Dieckmann and Couvet2004). For example, re-establishment of the monogamous white tailed sea eagle Haliaeetus albicilla in Scotland was slow because chance differences in the numbers of males and females led to a significant number of individuals failing to form pairs (Green et al., Reference Green, Pienkowski and Love1996). Unequivocal identification of the sex of individuals appears essential in reintroduction projects (Griffiths & Tiwari, Reference Griffiths and Tiwari1995; IUCN, 1998) but, when selecting founders, sex may be uncertain for young of most species and also for adults of monomorphic species. In such cases the strategy usually adopted is to collect individuals randomly from wild populations, hoping for a balanced sex ratio in the founding group. However, this strategy implies that sex ratio at birth and in demographic traits such as mortality and dispersal is not skewed.
Griffon vultures Gyps fulvus, a monogamous long-lived colonial scavenger, underwent a severe demographic decline in Western Europe from the end of the 19th century to the beginning of the 20th (Donázar, Reference Donázar1993), mainly due to human persecution and changes in agricultural practices and veterinary laws that reduced carrion availability (Terrasse, Reference Terrasse, Wilbur and Jackson1983). In France the griffon vulture disappeared in 1945 except in the Pyrenees. In Spain the species declined to c. 3,000 pairs in 1970 but increased during the 1980s to >18,000 in 1999 (Del Moral & Marti, Reference Del Moral and Marti2001). After five reintroduction programmes three colonies were re-established in southern France by 2006. Founders were mostly weak individuals rescued by centres in Spain and the French Pyrenees or from zoos. The sex of most was unknown before release. Roselaar (Reference Roselaar, Cramp and Simmons1979) showed that males and females were monomorphic but analyses were carried out on only 19 skins of adult individuals. More analyses on chicks and adults are needed to confirm the sexual monomorphism of this species. Additionally, sex ratio, sex-related mortality and movement are poorly known in populations of griffon vulture (Blanco & Martinez, Reference Blanco and Martinez1996). In this context, collecting weak individuals in populations to constitute founding groups for reintroduction may be a risky strategy if male and female life histories are different. There is therefore a need to study sex bias in griffon vulture life histories to improve the sampling strategy for founders.
We used a molecular sexing technique (Griffiths et al., Reference Griffiths, Double, Orr and Dawson1998) to investigate sex patterns in native and released populations of griffon vultures. Through long-term monitoring of wild-hatched and released individuals we examined (1) whether sex ratio is balanced in both wild-hatched and released groups, (2) if there are differences in biometrical measurements between sexes, and (3) sex bias in recoveries (i.e. dead individuals collected), movements (i.e. observations of individuals out of their home range), philopatry (i.e. recruitment of an individual in its birth colony) and recaptures (i.e. rescue of weak individuals) in both groups. We expected that if, in wild-hatched populations, sex ratio is balanced and demographic traits are not sex-biased, sex ratio in released groups should also be balanced.
Methods
We studied two wild-hatched populations of griffon vulture (one native colony in the French Pyrenees in Ossau and one reintroduced population settled in the south of the Massif Central in Causses) and four released groups (Navacelles, Baronnies, Verdon, Diois; Fig. 1). After a decrease in the late 1960s the population in Ossau increased to 114 pairs in 2005. In Causses, griffon vultures were first reintroduced in the beginning of the 1980s by the Parc National des Cévennes and the Ligue pour la Protection des Oiseaux, BirdLife France (Terrasse et al., Reference Terrasse, Bagnolini, Bonnet, Pinna, Sarrazin, Meyburg and Chancellor1994, 2004). Sixty-one individuals were released from 1981 to 1986. Since then, the population has increased continuously and reached 130-140 pairs in 2005. Almost all birds hatched in the wild in Causses were ringed in the nest (from 1982 to 2004, n = 674). In Ossau, a sample of individuals has been marked each year since 1993 (from 1993 to 2004, n = 432).
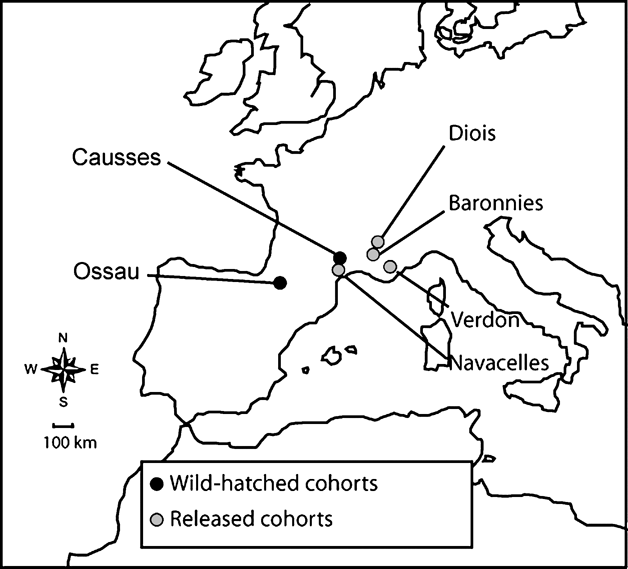
Fig. 1 Location of griffon vulture colonies studied in southern France.
In the locality of Navacelles, 46 km from Causses, 50 individuals were reintroduced from 1993 to 1997. Only one pair reproduced successfully between 1995 and 1998 and progressively all individuals left the site, which was abandoned in 1998 (Terrasse et al., Reference Terrasse, Sarrazin, Choisy, Clémente, Henriquet, Lecuyer, Pinna, Tessier, Chancellor and Meyburg2004). In Baronnies 61 birds were released between 1996 and 2001; in 2005, 53 breeding pairs were counted. In Verdon 91 birds were released between 1999 and 2005. Seventeen pairs bred in 2005. In Diois c. 60 birds have been released since 1999 but most of them bred in Baronnies. All released individuals were marked with a metal ring and an identification mark in the form of a combination of three or four coloured rings or a unique engraved ring.
Individuals' tissue samples were taken during ringing from 1993 onwards (Table 1). Growing feathers of 858 wild-hatched chicks were collected and preserved in 70% ethanol. Cubitus length of 515 chicks was measured, following Elosegui (Reference Elosegui1989), by the same technician at each colony. Additionally, chicks from the Ossau population were weighed. Blood samples were collected from 179 captive adults before release and conserved in a lysis buffer (0.1 M Tris-HCl, pH 8.0; 0.1 M EDTA, pH 8.0; 10 mM NaCl; 0.5% SDS). At the same time, biometrical measurements were taken (weight, tarsus length, bill height, head-bill length, flexed wing length; following Elosegui, Reference Elosegui1989) of 92 of the captive birds.
Table 1 Collected samples and number of sexed individuals in all colonies (Fig. 1).

Molecular sexing
We used a molecular sexing technique based on the detection of the coding chromodomain-helicase-DNA-binding gene (CHD gene; Ellegren, Reference Ellegren1996). The CHD gene is a conservative region in most avian genomes, present in two forms linked to the two sex chromosomes, Z and W, and differing in size. Both genes occur in females (the heterogametic sex in birds), whereas in males only the CHD-Z is present. DNA was extracted from 1998 to 2003 using three different methods in succession: the Chelex 100 method (Ellegren, Reference Ellegren, Hermann and Hummel1994), the standard phenol/chloroform extraction (Sambrook et al., Reference Sambrook, Fritsch and Maniatis1989) and the CTAB (Cethyl Trimethyl Ammonium Bromide) method (Kretzmann et al., Reference Kretzmann, Capote, Gautschi, Godoy, Donázar and Negro2003). In all cases we used a small clot (4 mm2) of thick blood or a 2 mm piece of the calamus. DNA extracted by the Chelex 100 method can be stored for some months at -20°C while DNA extracted by the standard phenol/chloroform and the CTAB methods can be stored for some years at −20°C. We amplified the CHD gene using the primer pair P2/P8, P2 (5'-TCT GCA TCG CTA AAT CCT TT-3') + P8 (5'-CTC CCA AGG ATG AGR AAY TG-3'). Results were validated with six individuals previously sexed by laparoscopy or karyotype analysis.
Statistical analysis
We tested if sex ratio was biased in wild-hatched populations and for all released groups at Navacelles, Baronnies, Verdon and Diois with χ2 tests. Cohort variation in sex ratio at birth or at release was tested using a logistic regression (R v. 2.1.0; Ihaka & Gentleman, Reference Ihaka and Gentleman1996). Yates’ continuity correction was applied for low number of released individuals. Biometrical measurements of wild-hatched chicks were highly age-dependent. We extracted residuals from linear regression between age and biometrical measures and we tested the effect of sex on these residuals using a gaussian general linear model (GLM). With a gaussian GLM we also tested if adults’ biometrical measurements varied between sexes.
We assumed that detectability was similar in both sexes. For each sexed individual we noted if it had been found dead or not and if it moved, at least once, from the colony where it was hatched or released. We also computed recapture events of weak wild-hatched individuals. We tested if those events were sex biased using logistic regression. We computed distances of movement with geographical coordinates of departure site and control site. As individuals prospected daily within a radius up to 32 km (Gault, Reference Gault2006) we considered only movements >32 km in our analysis. Moreover, in Causses, Baronnies and Verdon, information about philopatry was available. We then tested if sex had an effect on recovery, movement and philopatry (binomial GLM). We examined effects of sex on distance of movement with a gaussian GLM. We characterized the type of movement, i.e. ‘effective dispersal’ if individuals bred outside their birth or release colony and ‘prospecting’ if individuals were occasionally seen outside their home range. We tested the effects of sex on the type of dispersal using a binomial GLM. Finally, we computed age at recovery for wild-hatched birds (RAGE), time lag from release to recovery for released individuals (RAGE2), age at first movement for wild-hatched birds (MAGE), and time lag from release to first movement (MAGE2), and tested the effect of sex on these variables.
Results
Sex ratio and biometry
Overall sex ratio of wild-hatched populations was not different from 1:1 (Causses, n = 393, χ2 = 0.37, P = 0.54; Ossau, n = 294, χ2 = 0.0068, P = 0.93; Table 1). Sex ratio was not different between cohorts in Causses (χ2 = 3.09, df = 14, P = 0.99) or in Ossau (χ2 = 3.002, df = 8, P = 0.93). The sex ratio of each released group was not significantly biased (Navacelles, n = 37, χ2 = 0.24, df = 1, P = 0.62; Baronnies, n = 38, χ2 = 0.42, df = 1, P = 0.52; Verdon, n = 27, χ2 = 1.83, df = 1, P = 0.17; Diois, n = 43, χ2 = 0.58, df = 1, P = 0.44; Table 1). Moreover, no sex bias was detected for each released cohort in each site (all P >0.05).
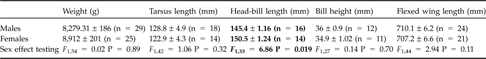
As expected, chick cubitus length (F 1,352 = 202.8, P <0.0001) and chick weight (F 1,213 = 123.5, P <0.0001) were significantly positively correlated with age at ringing. Chick biometrical measures were not affected by sex (cubitus length, F 1,146 = 1.65, P = 0.19; weight, F 1,146 = 0.004, P = 0.95). On the contrary, head-bill length was significantly lower in adult males than adult females (Table 2).
Table 2 Mean biometrical measurements, with SD, for adults caught during the study. Measurements that differed significantly between adult males and females are indicated in bold.
Recoveries, recapture, movement and philopatry
Among the 687 sexed wild-hatched individuals, 94 were recovered, 46 were rescued and 95 moved at least once. Sex had no effect on recovery rate (n = 687, χ2 = 0.15, df = 1, P = 0.69), recapture rate (n = 687, χ2 = 1.08, df = 1, P = 0.29) or movement rate (n = 687, χ2 = 0.75, df = 1, P = 0.38). Of the 145 released individuals that were sexed, 13 were recovered and 61 moved at least once. We did not detect any effect of sex on recovery rate (n = 145, χ2 = 3.71, df = 1, P = 0.06) or movement rate (n = 145, χ2 = 0.59, df = 1, P = 0.44).
Movement distances of wild-hatched birds were 54.6-3,930 km, with 71% of movements <500 km. Sex had no effect on distance moved (n = 91, F 1,89 = 2.8, P = 0.09). Among 393 sexed individuals hatched in Causses, 36 were philopatric. Sex had no effect on philopatry (n = 393, χ2 = 1.18, df = 1, P = 0.27). We did not find an effect of sex (n = 95, χ2 = 1.12, df = 1, P = 0.29) on the type of movement (effective dispersal vs prospecting). Movement distances of released individuals were 43-1,175.2 km with 95% of movements <500 km. As for wild-hatched populations, movement distance did not vary between sexes (n = 61, F 1,60 = 3.6, P = 0.08). Among 65 sexed individuals released in Baronnies and Verdon, 21 were philopatric. Sex had no effect on philopatry (n = 65, χ2 = 0.27, df = 1, P = 0.6) or on the type of movement (n = 61, df = 1, χ2 = 0.44, P = 0.5). Sex had no effect on age at recovery (RAGE, F 1,94 = 0.35, P = 0.55; RAGE2, F 1,13 = 2.97, P = 0.11) or on age at first movement (MAGE, F 1,101 = 0.56, P = 0.45; MAGE2, F 1,61 = 1.02, P = 0.31).
Discussion
In wild-hatched groups we found a sex ratio at ringing equal to 1:1, as expected in a monomorphic species (Fisher, Reference Fisher1930). We suspected that sex ratio at ringing was similar to sex ratio at fledging as recoveries before fledgling were not sex-biased (10 males and seven females). Using our data on measurements we confirmed that the species is monomorphic. We found that adult females had a longer head-bill than adult males while Mundy et al. (Reference Mundy, Butchart, Ledger and Piper1992) noted the opposite. However, we do not expect that this small difference would affect competitive abilities between sexes. We found no sex effect in recovery rate (frequency and age), movement rate (frequency, distance, type, age), philopatry or recapture of weak individuals in wild-hatched populations. Nevertheless, we considered recoveries to be a crude index of a combination of survival, dispersal and detectability, although any effect of sex on this index should be assessed through capture-mark-recapture (Lebreton et al., Reference Lebreton, Burnham, Clobert and Anderson1992) to discriminate between these processes.
Absence of sex bias in birth, mortality and dispersal could be explained by low competition between sexes and equal investment in reproduction by males and females. A skewed offspring sex ratio is expected when a difference between male and female offspring exists in their use of local resource (Clark, Reference Clark1978). For griffon vultures, competition for resources does not appear to be related to sex (Bosé & Sarrazin, Reference Bosé and Sarrazin2007). In the wandering albatross, however, a long-lived, monogamous and monomorphic species, male and female parents have different survivorships due to their quality and the sex of their offspring (Weimerskirch et al., Reference Weimerskirch, Lallemand and Martin2005). This may be due to their differential investment in parental care. However, both sexes of griffon vulture are known to invest in chick rearing (Mendelssohn & Leshem, Reference Mendelssohn, Leshem, Wilbur and Jackson1983). Sex-biased dispersal is supposed to be influenced by mating strategies (Greenwood, Reference Greenwood1980) but direction of the bias is hard to predict when cost of inbreeding is the same for both sexes (Perrin & Mazalov, Reference Perrin and Mazalov2000). Within the griffon vulture population around the Mediterranean no inbreeding and no differences in genetic diversity have been detected (Le Gouar et al., Reference Le Gouar, Rigal, Boisselier-Dubayle, Sarrazin, Arthur, Choisy, Hatzofe, Henriquet, Lecuyer, Tessier, Susic and Samadi2007).
Our study has therefore revealed that no sex bias occurred in any phase of the griffon vulture life cycle (i.e. birth, death, movement, philopatry and recapture). Consequently, sampling of rescued birds would not lead to sex-biased founding stocks. In the colonies in France the sex ratio of all released stocks was balanced and sex patterns in recoveries, movement and philopatry were not disturbed by reintroduction. As a result, no mating limitation and high reproductive success are expected in these founding populations because of their monogamous mating system (Legendre, Reference Legendre, Ferrière, Dieckmann and Couvet2004). However, the relationship between the growth of reintroduced populations and sex-ratio depends on their mating system. For instance, Komers & Curman (Reference Komer and Curman2000) found that in polygamous ungulate species population growth was positively influenced by a male biased sex ratio.
To conclude, we detected no sex bias in demographic parameters of griffon vultures hatched in the wild or in released populations, confirming the effectiveness of founder sampling to form sex-balanced founding stock in monogamous and sexually monomorphic species. However, the genetic and behavioural consequences of this strategy also need to be monitored.
Acknowledgements
This study results from collaboration with various organizations in charge of the griffon vulture reintroduction and conservation programmes: Parc National des Cévennes, Parc National des Pyrénées, Parc naturel régional du Vercors, LPO Grands Causses, LPO PACA, Association ‘Vautours en Baronnies’, Association ‘Vautours en Haute-Provence’, and Office National des Forêts. We owe special thanks to J.L. Pinna, D. Peyrusqué, J.M. Tabard and O. Lannès, who collected tissue samples. We thank J.M. Pericard for sexing griffon vultures using laparoscopy. We also thank J. Shykoff for support and advice, and B. Gautschi and S. Nadot for technical support. Financial support came from the Ministère de l'Ecologie et du Developpement Durable, Institut d'Ecologie Fondamentale et Appliquée (IFR101-CNRS) and from a Swiss National Science Foundation Grant 31-49477.96 to Dr Jürg Paul Müller and Professor B. Schmid.
Biographical sketches
Michela Bosé studies social feeding behaviour and intraspecific competition in griffon vultures. Pascaline Le Gouar is studying the impact of breeding habitat selection on the success of reintroduced populations through demographic, genetic and behavioural analyses and spatially explicit modelling. Christian Arthur, Jean Pierre Choisy, Sylvain Henriquet, Philippe Lecuyer and Christian Tessier monitor and manage griffon vulture colonies on a daily basis to improve their conservation. Josie Lambourdière and Muriel Richard work to improve molecular techniques on various species. François Sarrazin's main interests are the short- and long-term viability of restored populations.





