Introduction
In the face of a predicted global amphibian extinction crisis (Whittaker et al., Reference Whittaker, Koo, Wake, Vredenburg and Levin2013), the Amphibian Conservation Action Plan acknowledges that the best hope for some high-risk species is the establishment and management of captive populations (Gascon et al., Reference Gascon, Collins, Moore, Church, McKay and Mendelson2007; Wren et al., Reference Wren, Angulo, Meredith, Kielgast, Dos Santos and Bishop2015). Captive breeding, head-starting (a technique that involves raising early-stage amphibians in captivity before releasing them to the wild), and reintroduction programmes (collectively ex-situ conservation) are increasingly important management tools, both as insurance policies for species at risk of extinction in the wild and in reintroducing individuals to ecosystems where they have declined or been extirpated (Gascon et al., Reference Gascon, Collins, Moore, Church, McKay and Mendelson2007). The number of ex-situ programmes has expanded rapidly in recent years: Harding et al. (Reference Harding, Griffiths and Pavajeau2016) reported a 57% increase in amphibian species involved in conservation breeding and reintroduction programmes since 2007, and Biega et al. (Reference Biega, Greenberg, Mooers, Jones and Martin2017) listed 532 amphibian species (7% of all amphibian species) held ex situ, compared to 4% 5 years earlier (Conde et al., Reference Conde, Flesness, Colchero, Jones and Scheuerlein2011).
However, amphibians held ex situ are not always those with the greatest conservation need (Dawson et al., Reference Dawson, Patel, Griffiths and Young2016). Biega et al. (Reference Biega, Greenberg, Mooers, Jones and Martin2017) reported that although amphibians held in zoos are as threatened as their close relatives not found in zoos, the former occupy a broader range of habitats and possess larger spatial ranges than their closest relatives not held in zoos. Given that range-restricted specialist amphibians may face the greatest short-term extinction risk (e.g. Sodhi et al., Reference Sodhi, Bickford, Diesmos, Lee, Koh and Brook2008), this bias may be problematic.
There may be meaningful differences between species simply held in zoos and those involved in conservation breeding programmes. The ex-situ conservation organization Amphibian Ark (2017) helps ensure the suitability of species and institutions selected for breeding programmes through its Conservation Needs Assessment and Program Implementation tool, and zoos often select species for breeding programmes on the basis of recommendations from regional Amphibian Taxon Advisory Groups (Barber & Poole, Reference Barber and Poole2014). Characteristics of conservation breeding programmes include research on species biology to inform conservation efforts, captive assurance colonies, educational exhibits, and species destined for reintroduction or wild-to-wild translocation (including head-starting programmes; Harding et al., Reference Harding, Griffiths and Pavajeau2016). Zoos house species for a variety of reasons other than their threatened status (Bowkett, Reference Bowkett2014), and must consider cost, husbandry requirements, and visitor appeal (Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015), whereas species targeted for conservation breeding programmes often (albeit not always) face imminent threats in the wild (Conde et al., Reference Conde, Flesness, Colchero, Jones and Scheuerlein2011). Therefore, it would be useful to differentiate between species held in zoos and those actively involved in conservation breeding programmes.
We investigate this issue here. We followed an identical methodology to that of Biega et al. (Reference Biega, Greenberg, Mooers, Jones and Martin2017) but used a new dataset comprising only species that are currently bred for conservation purposes (i.e. not for medical reasons or general display in zoos) or involved in head-starting programmes. We tested how the same eight variables relating to extinction risk (IUCN status, habitat specialization, obligate stream-breeding, geographical range size, body size, and island, high-altitude and tropical endemism) vary between amphibian species involved in conservation breeding programmes and their close relatives not in such programmes. This analysis facilitates evaluation of how species involved in conservation breeding programmes compare to those in ex-situ holdings more generally, and how well conservation breeding programmes are targeting species of both immediate and future conservation concern.
Methods
We applied the methods described in Biega et al. (Reference Biega, Greenberg, Mooers, Jones and Martin2017) to focus specifically on species involved in conservation breeding programmes. We compiled a list of species in conservation breeding programmes, using the same list and criteria presented by Harding et al. (Reference Harding, Griffiths and Pavajeau2016). This comprised 213 species involved in conservation breeding programmes up to the end of 2013, 77 of which were initiated after 2007 (Harding et al., Reference Harding, Griffiths and Pavajeau2016). To test how extinction risk varies between amphibian species involved in conservation breeding programmes and their close relatives not in such programmes, we identified, on a phylogenetic tree, independent pairs of species that differed in the character of interest (contrasting species in breeding programmes with those that were not), and then examined how members of each pair differed with regard to extinction risk (Fig. 1). Because each pair (or ‘contrast’) was phylogenetically independent of every other pair, we could perform statistical tests (e.g. sign tests) and, using the phylogeny, construct phylogenetically corrected linear models in a multi-model inference framework (Ives & Garland, Reference Ives, Garland and Garamszegi2014). We could thus investigate which variables were most important in explaining the likelihood of a species being involved in a conservation breeding programme. In total we paired 130 species in conservation breeding programmes with their closest relatives not in such programmes, producing 111 independent contrasts. Our complete dataset is provided as Supplementary Material.
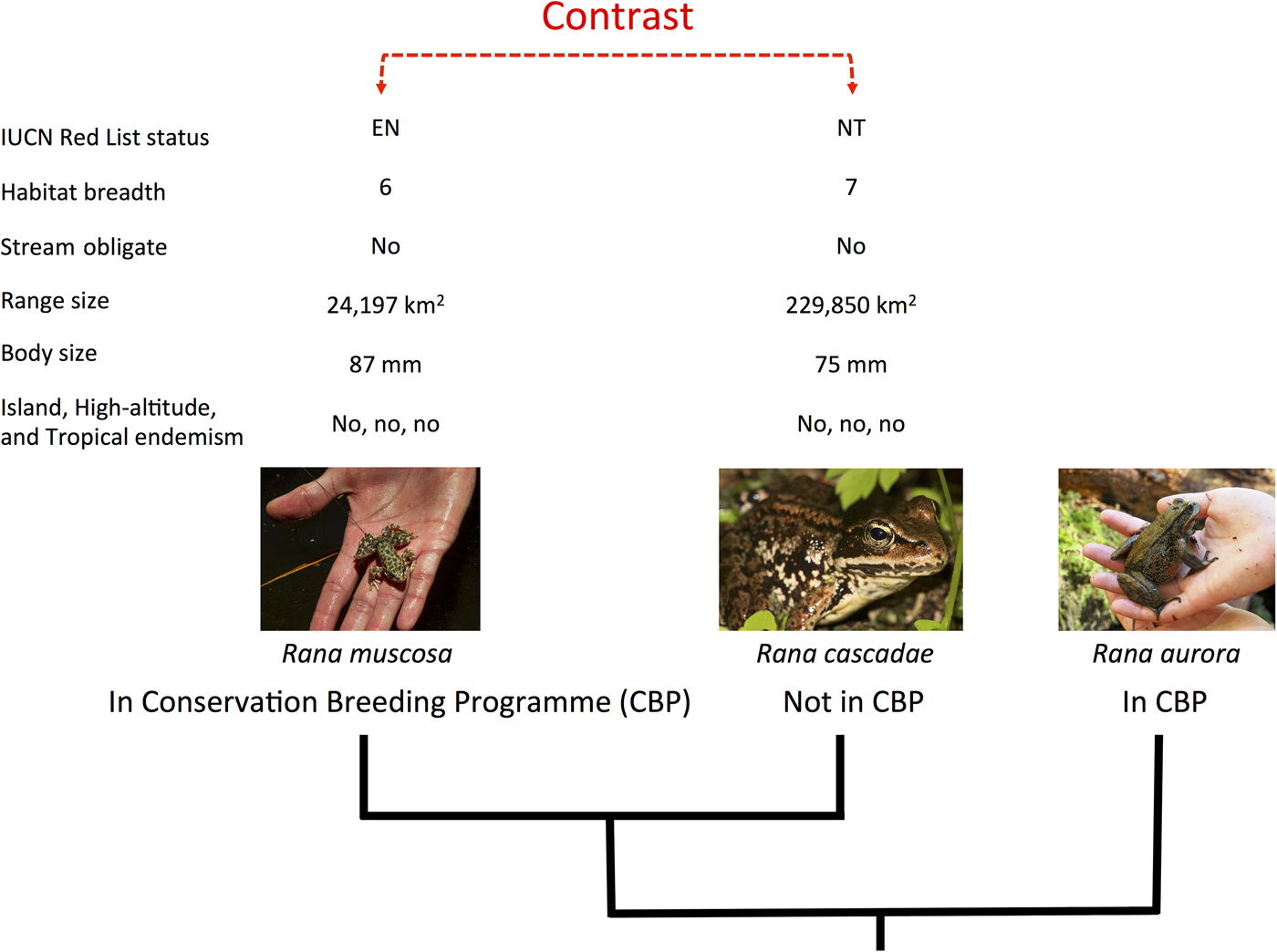
Fig. 1 The experimental design of our paired species analytical approach. Amphibian species in conservation breeding programmes were first paired to their closest relative(s) not involved in such programmes and then scored for eight variables relating to extinction risk (IUCN Red List status, habitat breadth, stream obligate status, geographical range size, body size, and island, high-altitude and tropical endemism). Differences between pairs were calculated and statistical tests (e.g. sign tests and randomization tests) were used to examine these differences. Species in conservation breeding programmes for which no monophyletic out-of-breeding programme relative could be identified (e.g. Rana aurora) were dropped from the analysis, to preserve statistical independence. Photograph credits (left to right): U.S. Geological Survey/Jenny Mehlow, Walter Seigmund, Dan Greenberg.
Species pair construction
Species in conservation breeding programmes were matched to their closest relatives (i.e. those with the smallest patristic distance) not involved in a conservation breeding programme, using the phylogenetic hypothesis from Pyron & Wiens (Reference Pyron and Wiens2011), with an updated taxonomy (Frost, Reference Frost2014). Congeners not in conservation breeding programmes may or may not have been held in a zoo. If a species was not found on the tree, it was added to the phylogeny if it had five or fewer congeners present on the tree, our cut-off for composite comparisons (see below). In many cases, a clade of several species in conservation breeding programmes shared the same closest relative not in a conservation breeding programme, or was matched with a clade of 2–5 species not in conservation breeding programmes. In these cases, species were grouped to produce ‘composite’ species for the contrast. For these species composites we used mean values for continuous variables and modal values for categorical variables. If no modal value could be determined, we discarded that variable from further analysis.
We retained all contrasts that (1) were true sister clades (i.e. we dropped paraphyletic contrasts), (2) included species for which there were data for at least four of our eight scoring variables, and (3) had five or fewer species in either of the two sister clades involved in the contrast (species in monotypic genera could still be involved in a contrast if they could be paired with a sister clade involving five or fewer species).
Selection and scoring of variables
We scored each species for eight variables known to relate to current and future extinction risk:
(1) IUCN threat score We scored a species as threatened if it was categorized as Data Deficient, Vulnerable, Endangered, Critically Endangered or Extinct in the Wild on the IUCN Red List (IUCN, 2016). Data Deficient species were considered to be threatened because they face, on average, a greater risk of extinction than fully assessed amphibians (Howard & Bickford, Reference Howard and Bickford2014). If conservation breeding programmes are selecting species based on conservation need, then species involved in such programmes will be more threatened than close relatives not involved in conservation breeding programmes, given that threatened species are, implicitly, of greater conservation priority.
(2) Habitat breadth We quantified habitat breadth by counting the total number of suitable habitats listed for each species based on the IUCN (2016) habitat classification scheme. Habitats of marginal or unknown suitability were excluded from these counts. If conservation breeding programmes are selecting species based on conservation need, then species involved in such programmes will have a narrower habitat breadth (i.e. will be more specialized) than their closest relatives not involved in conservation breeding programmes, based on the observation that a high degree of habitat specialization, and the associated low ecological tolerances and adaptability, directly correlate with extinction risk in amphibians (Williams & Hero, Reference Williams and Hero1998).
(3) Stream obligate status We scored a species as stream obligate if it was listed under the ‘stream, river, or creek’ habitat classification (coded as 5.1 for permanent habitats and 5.2 for temporary habitats) as its sole aquatic habitat (IUCN, 2016). If conservation breeding programmes are selecting species based on conservation need, then species involved in such programmes will be more reliant on stream habitats than their close relatives not involved in conservation breeding programmes, given that dependence on riparian habitats has been identified as one of the key correlates of amphibian threat status (Lips et al., Reference Lips, Reeve and Witters2003; Stuart et al., Reference Stuart, Chanson, Cox, Young, Rodrigues, Fischman and Waller2004), species in these habitats being particularly prone to infection by the fungal disease chytridiomycosis (Kriger & Hero, Reference Kriger and Hero2007).
(4) Geographical range size Geographical range sizes (in km2) were calculated in R v 3.3.3 (R Development Core Team, 2015) for each species in our sample, using georeferenced spatial polygons depicting the current known distribution of the species within its native range. These polygon shapefiles for each species are freely available for download from IUCN (2016). If conservation breeding programmes are selecting species based on conservation need, then species involved in such programmes will possess smaller geographical ranges than close relatives not involved in conservation breeding programmes, given that range-restricted amphibians are at greater risk of global extinction (Sodhi et al., Reference Sodhi, Bickford, Diesmos, Lee, Koh and Brook2008) and are inherently more at risk from localized habitat destruction and fragmentation (Pimm et al., Reference Pimm, Russell, Gittleman and Brooks1995; Purvis et al., Reference Purvis, Gittleman, Cowlishaw and Mace2000).
(5) High-altitude endemism We scored a species as a high-altitude endemic if it was recorded by IUCN (2016) as living exclusively at > 1,000 m altitude. This 1,000 m criterion is based on delimitations of high-altitude life-zones defined in Spehn & Körner (Reference Spehn, Körner, Huber, Bugmann and Reasoner2005). If conservation breeding programmes are selecting species based on conservation need, then montane species will be better represented than non-montane close relatives, given that high-altitude amphibian species face increased risks from infectious diseases (Lips et al., Reference Lips, Reeve and Witters2003) and climate change (Pounds et al., Reference Pounds, Fogden and Campbell1999).
(6) Island endemism We scored a species as being an island endemic if it occurred exclusively in island ecosystems, based on IUCN (2016) range maps. If conservation breeding programmes are selecting species based on conservation need, then island endemic amphibians will be better represented than non-island close relatives, given that island endemics inherently possess restricted spatial ranges (see above), and the biogeographically isolated nature of these endemics often enhances extinction risk (Fordham & Brook, Reference Fordham and Brook2010).
(7) Tropical endemism A species was scored as a tropical endemic if it occurred exclusively within one or more of the three major tropical zoogeographical regions (Neotropical, Afrotropical, and Oriental; Cox, Reference Cox2001), based on IUCN (2016) range maps. If conservation breeding programmes are selecting species based on conservation need, then species restricted entirely to tropical zoogeographical zones will be better represented in such programmes than non-tropical close relatives, given that tropical species face greater environmental pressures and higher extinction risks, on average, than temperate species (Vamosi & Vamosi, Reference Vamosi and Vamosi2008).
(8) Body size We obtained body size measurements from Biega et al. (Reference Biega, Greenberg, Mooers, Jones and Martin2017), which in turn sourced data largely from a comprehensive amphibian life-history dataset (Costa et al., unpubl. data), further augmented by data from the wider literature (see Supplementary Material for all literature sources used). Snout–vent lengths were used for Anurans, and total body length was used for Caudates and Caecilians. We hypothesize that species held in conservation breeding programmes will be larger than close relatives not involved in breeding programmes, given (1) the weak positive correlation between body size and extinction risk in amphibians (Lips et al., Reference Lips, Reeve and Witters2003; Sodhi et al., Reference Sodhi, Bickford, Diesmos, Lee, Koh and Brook2008), and (2) biases towards larger bodied species found in ex-situ holdings for other taxa.
Statistical analysis
To ensure the sample of species used in our paired analysis was representative of all species involved in conservation breeding programmes, we conducted a series of Z-tests (Zar, Reference Zar1999) comparing the mean scores of all variables for the 130 species in our sample with the 209 unique species listed by Harding et al. (Reference Harding, Griffiths and Pavajeau2016). Species were grouped by taxonomic order. We then determined differences between pairs of species included and not included in conservation breeding programmes. Differences in binary variables (threat status, stream obligate status, and the three measures of endemism) were assessed using simple sign tests (Zar, Reference Zar1999), and differences in continuous variables (habitat breadth, spatial range, and body size) were assessed using randomization tests. The randomization tests evaluated the mean difference in our matched-pair comparisons against the null distribution produced by randomizing observed differences with an equal probability of being positive or negative 10,000 times (Felsenstein, Reference Felsenstein1985). This created an expected distribution of differences under the assumption of no predictive power of in-programme status for the contrast. The mean observed difference for each variable could then be compared to its null distribution to determine its significance.
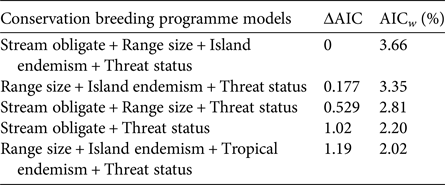
We investigated which variables were most important in explaining the likelihood of being involved in a conservation breeding programme, using a multi-model inference approach comparing models that included various combinations of all eight variables. We modelled the probability of a species being in a conservation breeding programme (1 or 0) using logistic regression. We compared all species used in the contrasts but assessed each species independently rather than using modes or means of traits for contrasts of several species. This resulted in a dataset of 362 species (209 in conservation breeding programmes, and 153 not in conservation breeding programmes). To facilitate a valid comparison of all factors, we removed species missing any of the eight scoring variables. We analysed all possible model combinations, as generalized linear models with a Bernoulli error distribution, to assess what combination of factors best explained the probability of a species being held in a conservation breeding programme. Model selection was based on Akaike information criterion (AIC) scores, and the importance of each predictor variable was based on cumulative AIC weights across all models. All analyses were completed in R, using code available upon request from Biega et al. (Reference Biega, Greenberg, Mooers, Jones and Martin2017).
Results
Z-tests indicated that our sample of species in conservation breeding programmes was representative of all Anurans and Caudates in such programmes. However, as only one Caecilian was included in our sample, Gymnophiona was found to be unrepresentative for four of the variables (P < 0.05). Following the rules for contrasts, the Caecilian contrast was removed from the analysis.
In contrast to zoo holdings more generally, we found that species involved in conservation breeding programmes are more threatened globally than their close relatives not involved in such programmes (P = 0.05). Furthermore, we found no significant difference between sister species for any of the other seven threat correlates (all P > 0.05, Table 1). These patterns were supported by multi-model inference methods in which threat status was the most highly weighted predictor of being part of a conservation breeding programme across models, followed by range size, stream obligate status, and island endemism (Table 1). Model selection results, including the five most parsimonious models and model-averaged variable coefficients, are in Tables 2 & 3.
Table 1 Contrasts between species involved in conservation breeding programmes and their closest relatives not involved in such programmes, and the same contrasts for global zoo holdings in general (Biega et al., Reference Biega, Greenberg, Mooers, Jones and Martin2017). Difference shows differences in positive (+) and negative (−) values between in breeding programmes/zoo holdings and not in breeding programmes/zoo holdings species pairs for categorical variables, and ratio differences between these pairs for continuous variables. P (n) indicates the probability for these tests, with sample sizes in parenthesis. Bold entries indicate significant differences between pairs (P ≤ 0.05). ΣAICw shows the relative importance of variables from multivariate analysis as indicated by cumulative Akaike weight, with asterisks denoting the top three variables by weight.
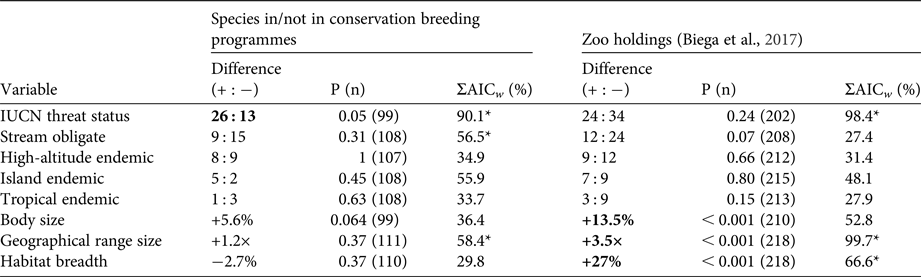
Table 2 Results of generalized linear model analyses determining the relative importance of eight traits in explaining the likelihood of a species being involved in a conservation breeding programme, with model-averaged logit coefficients (Bavg), standard errors (SE), and lower and upper 95% confidence intervals.

Table 3 Top five multivariate general linear models, based on the Akaike information criterion (AIC), for predicting the likelihood of an amphibian species being involved in a conservation breeding programme. ΔAIC indicates the difference in the AIC value from the top model, and the Akaike weight (AICw) provides a relative weight of evidence for each model.
Discussion
It is unsurprising that conservation-focused breeding programmes are targeting threatened species, given their general purpose is to target species facing imminent extinction in the wild. The initiation of breeding or reintroduction programmes is frequently tied to regional, national or subnational environmental and legislative objectives for native species. Although some species in these programmes are not listed as globally threatened, they may still be targeted by conservation breeding programmes because of more local threats; for example, the northern leopard frog Lithobates pipiens (a species bred by the Vancouver Aquarium) is categorized as Endangered in British Columbia but as Least Concern globally (IUCN, 2016). Other important reasons zoos choose to hold non-threatened species include financial and logistical constraints (Bowkett, Reference Bowkett2014). Nineteen non-threatened species on our list are bred for conservation research, possibly to gain husbandry knowledge that could be applied to holding imperilled relatives in the future.
Although birds, mammals and amphibians kept in zoos have larger body sizes than their closest relatives not in zoos (Martin et al., Reference Martin, Lurbiecki, Joy and Mooers2014; Biega et al., Reference Biega, Greenberg, Mooers, Jones and Martin2017), amphibians involved in conservation breeding programmes were no different in size to their close relatives. Smaller-bodied amphibians may be more attractive to zoos with limited space, but larger-bodied, attractive and charismatic species may be more desirable for zoo visitors (Frynta et al., Reference Frynta, Šimková, Lisšková and Landová2013). However, zoo-reared amphibians destined for re-release are often kept in specialist biosecure facilities, isolated from other holdings and not seen by visitors. Visitor expectations may therefore play a lesser role when choosing species for conservation breeding programmes. Only 46% of the species in conservation breeding programmes on the list used in our analysis are exclusively zoo-held; another 46% are raised in specialist facilities run by government or non-government agencies, and the remaining 8% are held within both (Harding et al., Reference Harding, Griffiths and Pavajeau2016).
Given the similar range sizes and habitat breadths of amphibians in conservation breeding programmes and their close relatives, it seems there are meaningful differences between amphibian species selected for zoo holdings generally and those selected for conservation breeding programmes (see Table 1 for a direct comparison). Biases found in global ex-situ species holdings towards wide-ranging habitat generalists are not reflected in the species selected for conservation breeding programmes. This is good news: conservation breeding programmes are targeting species facing both immediate and medium-term extinction risks.
Although captive breeding can be a key component of imperilled species recovery, it should be acknowledged that some amphibian species fail to thrive in captivity (Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015), and that a species’ suitability for a breeding programme must be considered before its establishment. Our modelling framework did not identify trends in specific traits associated with conservation breeding programmes; however, a study examining life history traits amongst successful and unsuccessful captive-bred species could reveal traits associated with amenability to captivity.
In summary, species involved in conservation breeding programmes are more threatened than their closest relatives not bred purely for conservation purposes. Although this analysis has no bearing on the success of these programmes (this was evaluated in Harding et al., Reference Harding, Griffiths and Pavajeau2016), it highlights important differences between amphibians held in zoos as a whole and those actively managed for ex-situ conservation. We encourage continued prioritization of species facing increased extinction risk for conservation breeding programmes, but emphasize that species’ suitability for such programmes must be assessed on a case-by-case basis.
Acknowledgements
We thank an anonymous reviewer from a previous study for suggesting we look specifically at conservation breeding programmes for amphibians; Gemma Harding and colleagues for providing open-access data on species held in conservation breeding programmes; and Dan Greenberg, Arne Mooers and two anonymous reviewers for constructive comments. This work was supported by the Natural Sciences and Engineering Research Council of Canada through CREATE, Discovery, and CGS-M grants. The dataset contributed by Gabriel Costa was supported by grants from the National Science Foundation (DEB 1136586), the Federal Agency for Support and Evaluation of Graduate Education (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; PVE 018/2012), and the National Council for Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico; 302297/2015-4, 201413/2014-0).
Author contributions
AB conceived and designed the project and collected the data. AB and TEM analysed the data and wrote the paper. Both authors edited and approved the final text.
Biographical sketches
Alannah Biega’s research interests include prioritizing species for ex-situ management and exploring the role of zoos in modern species conservation. Tom Martin specializes in comparative work on the role of ex-situ conservation by zoos.






