Introduction
Plants play a key role in maintaining healthy ecosystems and they provide essential habitat for animal species (CBD, 2011). They also provide vital resources, such as food, timber and fibres, and many plants have great cultural importance (CBD, 2011). Plant conservation is increasingly important in the face of the many threats to plant diversity, including climate change, land-use change, over-exploitation, pollution and invasive alien species (CBD, 2011).
The genus Dracaena L. comprises c. 100 species and ranges from Macaronesia to Australia, with one species in the Neotropics (Bos, Reference Bos and Kubitzki1998). Taxonomic revisions are being carried out throughout its range and threatened narrow endemics of the genus have been described from Africa (Mwachala et al., Reference Mwachala, Cheek, Fischer and Muasya2007; Mwachala & Cheek, Reference Mwachala and Cheek2012; Mwachala & Fisher, Reference Mwachala and Fisher2013) and South-east Asia (Wilkin et al., Reference Wilkin, Suksathan, Keeratikiat, Van Welzen and Wiland-Szymańska2012), with c. 46 species described for Africa (Mwachala & Fisher, Reference Mwachala and Fisher2013).
Among Dracaena, the dragon tree group, which has a unique and distinctive appearance (Plate 1), comprises 10 arborescent species (Wilkin et al., Reference Wilkin, Suksathan, Keeratikiat, Van Welzen and Wiland-Szymańska2012), all growing in seasonally arid climates with annual rainfall of 200–500 mm and mean temperatures of 18–20 °C (Marrero et al., Reference Marrero, Almeida and Gonzàlez-Martìn1998; Adolt & Pavlis, Reference Adolt and Pavlis2004). These species exhibit a biogeographical disjunction, regarded by Adolt & Pavlis (Reference Adolt and Pavlis2004) as ‘a relic representation of the Mio-Pliocene Laurasian subtropical flora’. Four species are known in South-east Asia (Dracaena cambodiana Pierre ex Gagnep., possibly conspecific with D. cochinchinensis (Lour.) S. C. Chen, D. jayiana Wilkin & Suksathan, and D. yuccifolia Ridl.), two in Macaronesia (D. draco L. and D. tamaranae Marrero Rodr. et al.), two in Africa (D. ellenbeckiana Engl. and D. ombet Heuglin ex Kotschy & Peyr., incl. D. schizantha Baker; Thulin, Reference Thulin and Thulin1995), one in the Arabian Peninsula (D. serrulata Baker, sometimes considered a conspecific of D. ombet), and one endemic to Socotra (D. cinnabari Balf. f.).

Plate 1 Nubian dragon tree Dracaena ombet in vivo in Gebel Elba National Park, Egypt (Fig. 1). (a) Healthy mature tree. (b) Dried tree as a result of drought.
The Nubian dragon tree Dracaena ombet, a flagship species (Vincent, Reference Vincent2008) of the Afromontane areas of north-east Africa, is found in bushland and woodland on mountain slopes facing the Red Sea, generally at 1,000–1,800 m (Thulin, Reference Thulin and Thulin1995). The main population occurs in the Gebel Elba Mountains in south-east Egypt (Ghazali et al., Reference Ghazali, El Baily, Dora, Arkeeb, Aoud and Ossman2008) and additional populations are found on Mount Erkowit in Sudan, on the escarpments of the Eritrean Mountains, and in Somaliland, Somalia and Ethiopia (Kassas, Reference Kassas1956; Friis, Reference Friis1992; Thulin, Reference Thulin and Thulin1995; Bos, Reference Bos, Edwards, Demissew and Hedberg1997), with a few scattered populations in the Horn of Africa (Audru et al., Reference Audru, Cesar, Forgiarini and Lebrun1987; Friis & Lawesson, Reference Friis and Lawesson1993; Magin, Reference Magin1999).
D. ombet is categorized as Endangered on the IUCN Red List (WCMC, 1998), based on an assessment made using a now outdated set of criteria (v. 2.3; IUCN, 1994). A survey of the Gebel Shindeeb population in the southern part of Gebel Elba National Park conducted by El Azzouni (Reference El Azzouni2003) revealed that it comprised only mature plants, many of which appeared unhealthy as a result of drought conditions, attack by a parasitic pest, and/or disease. D. ombet is extirpated in Erowit, in northern Sudan, one of the few areas where the species had survived in that country (El Azzouni, Reference El Azzouni2003). These observations point to a rapid decline of the Nubian dragon tree in at least part of its range. In south-eastern Egypt the species generally occurs in remote, high-elevation areas that are difficult to access, and no reliable information is available regarding its ecology and conservation status in this part of its range.
A survey and monitoring project was launched in 2007 to support a conservation action plan for the Egyptian population of D. ombet (Ghazali et al., Reference Ghazali, El Baily, Dora, Arkeeb, Aoud and Ossman2008). A survey of its distribution in Gebel Elba National Park was undertaken during October 2007–March 2009. Comprehensive geographical information system-based mapping and eco-geographical surveys were used to identify areas of current distribution and to assess the status and health of the Gebel Elba population. This provides the first accurate baseline for developing a conservation strategy to protect D. ombet in an effort to prevent its extinction within the Park. Furthermore, the survey generated the data necessary for a preliminary IUCN risk assessment at the regional level, using IUCN Criteria v. 3.1 (IUCN, 2012). An updated Red List assessment is also provided for D. ombet throughout its range.
Study area
Gebel Elba National Park (c. 35,600 km2) is located in south-eastern Egypt (Fig. 1), immediately north of the border with Sudan. The Park includes a cluster of coastal mountains overlooking the Red Sea, the most prominent peaks being Gebel Kam Erba (1,374 m), Gebel Shellal (1,409 m), Gebel Elba (1,435 m), Gebel Shendodai (1,526 m) and Gebel Shendeib (1,911 m). Although not the highest peak, Gebel Elba has the highest levels of precipitation because it is closest to the sea and faces the north-east winds. The Gebel Elba region is recognized as a biodiversity hotspot, located among the northern outliers of the Horn of Africa hotspot (Conservation International, 2013). Many Afrotropical elements reach their northern limits at Gebel Elba, contributing to a level of biological diversity that is unmatched anywhere else in Egypt (Ghazali et al., Reference Ghazali, El Baily, Dora, Arkeeb, Aoud and Ossman2008).

Fig. 1 The study area in Gebel Elba National Park, Egypt. The filled circle on (a) shows the location of the Gebel Elba region. The rectangle on (b) shows the location of the eight wadi surveyed (c). ADH, Aedieb Hills; ADB, Aedieb; AKW, Akaw; ART, Aretri; DRW, Darawina; MFY, Marafay; SHT, Shtet; TWL, Tawella.
In the southern part of the Egyptian desert the mean monthly temperature is 20.9 °C in January and 31.9 °C in August (range 16.8–37.1 °C; NCDC, 2013). Mean annual rainfall in the region is < 50 mm but in Gebel Elba the prevailing south-east winds bring mist and clouds to the mountain slopes (Abu Al-Izz, Reference Abu Al-Izz1971), where orographic precipitation produces up to 400 mm of rainfall per year. These localized conditions have facilitated the establishment of a rich and diverse flora (350 species), including several tree species that grow at higher elevations (Ghabbour, Reference Ghabbour1997).
Methods
The distribution of D. ombet in Gebel Elba National Park was recorded through field surveys conducted with the participation of the local community (Ghazali et al., Reference Ghazali, El Baily, Dora, Arkeeb, Aoud and Ossman2008). Stands of the tree were found on three mountain massifs within the Park: Gebal Kam Erba, Gebel Elba and Gebel Shendeib (Fig. 1). Only populations on the most northern massif, Gebel Elba, were surveyed in detail because of the proximity of the area to the Sudanese border, which is unsafe to visit. The following parameters were recorded: number of trees, composition of associated plant communities, and level of grazing along the mountain's elevational gradient. The precise location of trees was recorded using a global positioning system (GPS) unit. Diameter at breast height (DBH) and total tree height were recorded for each individual, and each tree was categorized as healthy (> 70% of the tree parts were living), moderately healthy (35–70%), unhealthy or weak (1–35%) or dead (0%). All living trees were marked with an aluminium tag bearing a unique number, for further monitoring. The age structure of the Gebel Elba Mountain population was recorded along with phenological status. Branching attributes, including branch height and number, were also recorded. To evaluate regeneration status we recorded the population size at each of four stages of maturity: seedlings, sprout shoots, saplings and mature trees. Seed dispersal and seedling survival in relation to distance from the mother plant were estimated by counting the number of seedlings and saplings and recording their distance from the presumed mother plant.
Data were analysed and distribution maps were generated using ArcView v.3.2 (ESRI, Redlands, USA), facilitating analysis of the spatial distribution of trees and determination of the areas of highest species density (population hotspots). For the preliminary assessment of risk of extinction we calculated the Extent of Occurrence (EOO), defined by IUCN (2013a) as the area contained within the shortest continuous boundary that can be drawn to encompass all the known, inferred or projected sites of present occurrence of a taxon, excluding cases of vagrancy, and the Area of Occupancy (AOO), defined as the area that is occupied by a taxon within the EOO, excluding cases of vagrancy. IUCN recommends using a grid cell 2–3 km2 when applying Criterion B (geographical range; Callmander et al., Reference Callmander, Schatz, Lowry, Laivao, Raharimampionona and Andriambololonera2007; IUCN, 2013a). However, the populations of D. ombet are so scattered that a global AOO is not representative, especially as large portions of the Gebel Elba population are known to be unhealthy. We therefore used Criterion A, quantifying population size for a preliminary assessment of risk of extinction. To estimate the past and present distribution of D. ombet at a regional scale we recorded the locality of each tree in the wadi of Gebel Elba. We estimated the reduction in population size since 1997, using data for both living and dead trees (sensu Criterion A, IUCN, 2012).
Results
Distribution and population structure
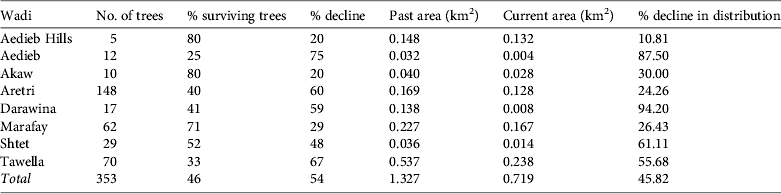
The Nubian dragon tree was detected in 13 wadi, eight of which, located in the Elba Mountain massif, were surveyed in detail (Fig. 1); the remaining five are located near the Sudanese border. The c. 353 trees recorded in the eight wadi on Elba Mountain (Table 1) occurred at elevations of 450–950 m. Of the 46% (161 individuals) that were still alive, only 27% (96 individuals) were healthy. A majority of the trees were dead in all of the wadi studied, with the exception of wadi Marfay, where 71% (c. 44 individuals) were living (Table 1). The north-east of Gebel Elba Mountain (Tawella and Aretri wadi; Fig. 1) has the largest Dracaena woodland within the Park, with > 210 trees. However, there is evidence that these sites suffer from drought and unsuitable climatic conditions, which may account for the observed decline of > 60% in the populations that occur there, indicating that they will probably be lost in the near future. DBH measurements (Fig. 2) indicate an imbalanced age structure in populations of D. ombet on Gebel Elba Mountain, with c. 90% of the recorded individuals categorized as mid-age trees (DBH 35–140 cm) and < 1% (two individuals) categorized as young trees (DBH < 35 cm).
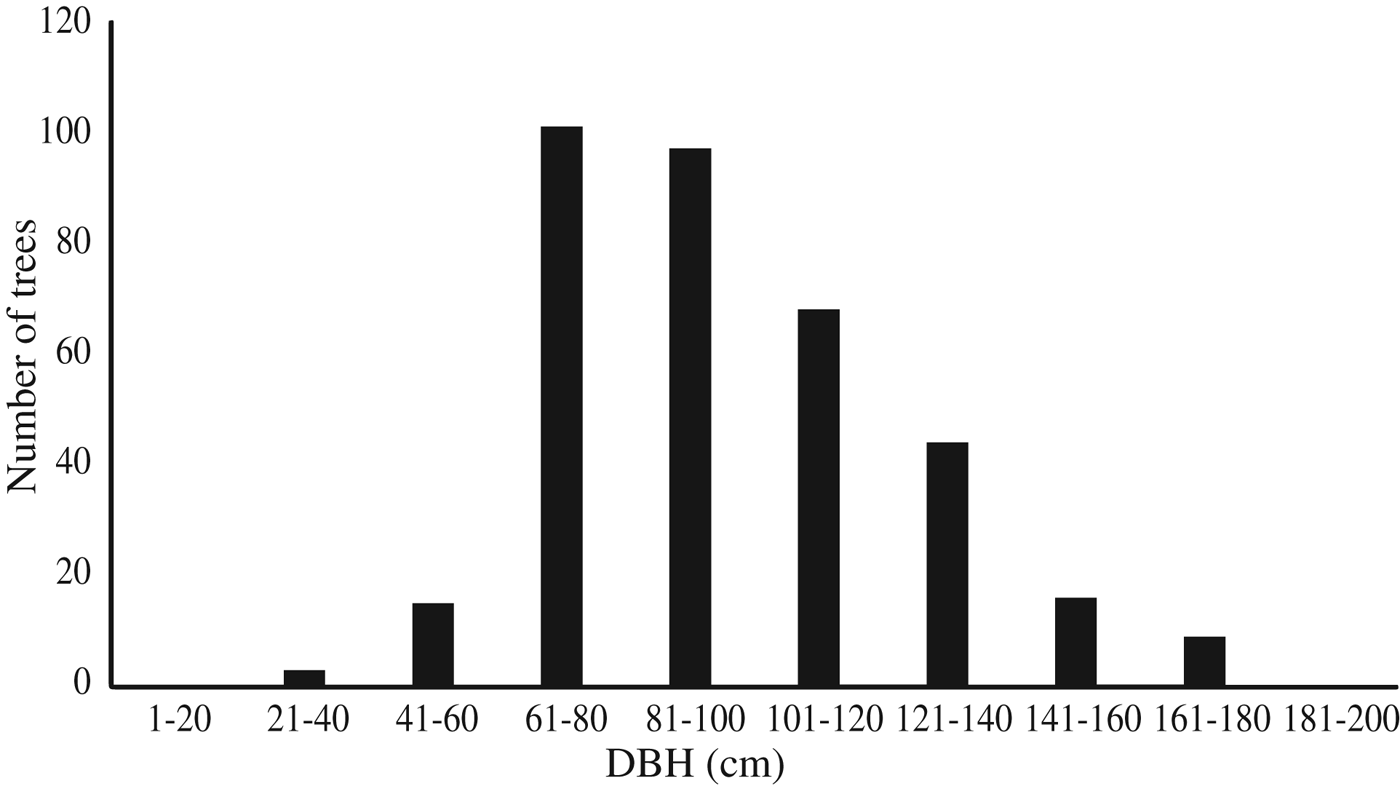
Fig. 2 Number of individuals of the Nubian dragon tree Dracaena ombet in each DBH class.
Table 1 Results of our survey of populations of Nubian dragon tree Dracaena ombet in eight wadi of the Gebel Elba Mountain in south-east Egypt (Fig. 1), with wadi name and location, number of trees, percentage of surviving trees, percentage decline, past and current areas of distribution, and percentage decline in distribution.
IUCN Red List assessment at regional and global scales
The EOO of D. ombet was calculated for the entire population within Gebel Elba National Park. Taking into account the isolated Shendieb Mountain (located c. 20 km from the wadi surveyed at Gebel Elba), the EOO is c. 116 km2. Because none of the area between these massifs is suitable for D. ombet, we also calculated an EOO excluding the small population on Shendieb Mountain, which is c. 24 km2, or 0.3% of the area of the Park. To apply Criterion A (population reduction) we calculated the distribution of the entire population of the survey (present and past, by including dead trees). Our estimate suggests that D. ombet occupied an AOO of 1.327 km2 as recently as 1997 but now occupies only 0.719 km2, indicating a loss of 45.9% of its range in the Gebel Elba Mountains (Table 1). As almost no regeneration is occurring and only 25% of the trees appear to be healthy we predict that all subpopulations are likely to disappear within a decade (i.e. within the time frame covered by Criterion A; IUCN, 2012). We estimate that D. ombet is likely to decline by > 80% in this time frame and consequently we categorize D. ombet as Critically Endangered under Criterion A3c (IUCN, 2012).
Discussion
The flagship species D. ombet (Vincent, Reference Vincent2008) has a fragmented distribution throughout the Gebel Elba massif, a pattern resembling that seen in other species of dragon tree, such as the Arabian dragon tree D. serrulata, which grows in scattered populations on the western and southern escarpments of the Arabian Peninsula (Llewellyn et al., Reference Llewellyn, Hall, Miller, Al-Abbasi, Al-Wetaid and Al Harbi2010). This type of distribution pattern reflects the ecology of these plants, which are adapted to specific edaphic and ecological conditions, such as limestone karsts (e.g. D. jayniana in Thailand; Wilkin et al., Reference Wilkin, Suksathan, Keeratikiat, Van Welzen and Wiland-Szymańska2012). Pressure from human activities has relegated most dragon trees to inaccessible sites, such as steep cliffs in the Canary Islands and Morocco, where D. draco persists today. The highest percentage of healthy individuals of D. ombet in Gebel Elba National Park is found on steep slopes of the Aedieb Hills, on solid basement rock with extensive cracks and fissures. These relicts of the Mio-Pliocene Laurasian subtropical forests are now rare, partly as a result of the climatic changes of the late Pliocene that caused desertification in North Africa (Quetzel, Reference Quetzel1978; Mies, Reference Mies and Dumont1996), which explains why D. ombet is now restricted to a narrow set of microhabitats.
The population of D. ombet on Gebel Shindeeb contains a high proportion of adult individuals, most of which are unhealthy. Extended periods of drought combined with high temperatures are clearly inhibiting regeneration (El Azzouni, Reference El Azzouni2003). Populations on the eastern slopes of Okoam Wahadel in Erkwit, Sudan, are facing similar challenges (Mohammed, Reference Mohammed2004), as are those of D. serrulata in the Najran Mountains of western Saudi Arabia (Llewellyn, Reference Llewellyn2009). Likewise in Socotra D. cinnabari shows low levels of regeneration and a population structure skewed towards mature individuals (Adolt & Pavlis, Reference Adolt and Pavlis2004). These marginal climatic conditions coupled with increasing human pressure are important factors affecting the status of the Nubian dragon tree in the Elba Mountains. The population decline is closely correlated with the periods of drought that have affected the area since 1950, especially during the 1960s–1980s (Hobbs, Reference Hobbs1992). Healthy populations of D. ombet are found only in inaccessible areas, in particular the Aedieb Hills. Attorre et al. (Reference Attorre, Francesconi, Taleb, Scholte, Saed, Alfo and Bruno2007) recorded regeneration and a healthy population of D. cinnabari at higher altitudes in Socotra, at similarly inaccessible sites that receive adequate rainfall.
All of the African dragon tree species are categorized as threatened on the IUCN Red List (2013b) but all except one of these assessments, that for D. cinnabari, are based on the outdated v. 2.3 of the Red List Categories and Criteria (IUCN, 1994) and need to be updated using newly compiled data and v. 3.1 of the Categories and Criteria (IUCN, 2012). The Arabian Plants Red List authority has recategorized D. cinnabari as Vulnerable (using criteria B2ab[iii]) based on data from Adolt & Pavlis (Reference Adolt and Pavlis2004) and Attorre et al. (Reference Attorre, Francesconi, Taleb, Scholte, Saed, Alfo and Bruno2007). D. ombet is categorized as Endangered (based on criteria A1cd; WCMC, 1998) but at a regional level in Gebel Elba National Park we assess it to be Critically Endangered under criterion A3c, based on IUCN (2012). Our study shows that populations are scattered and several are declining, with only 27% healthy trees and almost no regeneration. The skewed age class structure of these populations suggests that the Nubian dragon tree is facing extinction in the wild. At a global scale we project that D. ombet is facing a similar situation throughout its range. Based on our results and other available information we regard the Nubian dragon tree to be globally Endangered (based on criterion A3c), using v. 3.1 of the IUCN Red List Categories and Criteria (2012).
It seems likely that a major cause of the decline in the extent and quality of the woodland habitats in which D. ombet occurs is the gradual drying of this part of southern Egypt (Springuel, Reference Springuel2006). To support the development of a programme to conserve D. ombet in Africa, comprehensive field studies are required to assess the current status of populations at all known localities for which updated field data are lacking, especially those in regions such as Bale Mountain in Ethiopia, Hargesia in Somaliland, Goda and Hemed Mountains in Djibouti, and Erkwit in Sudan. Improving data on the distribution of this species will facilitate more accurate distribution modelling (Guisan et al., Reference Guisan, Graham, Elith and Huettmann2007). Because montane plants are particularly susceptible to the effects of climatic change (Walther et al., Reference Walther, Post, Convey, Menzel, Parmesan and Beebee2002; Thuiller et al., Reference Thuiller, Lavorel, Araújo, Sykes and Prentice2005), detailed studies to predict the response of D. ombet to this pressure are essential for in situ conservation planning and action. Developing a better understanding of the ecology of D. ombet and implementing an appropriate conservation management strategy would be further supported by population genetic studies. It will also be necessary to clarify the taxonomic status of D. ombet with regard to D. serrulata. Finally, ethno-botanical research would also be of value to understand the socio-economic importance of this species. These complementary lines of research are needed to develop and reinvigorate a traditional conservation system in which D. ombet would be featured as a flagship species to promote biodiversity conservation and sustainable use in the threatened areas where the Nubian dragon tree is found (Hall et al., Reference Hall, Neale, Al-Abbasi and Miller2010).
Acknowledgements
We thank the communities of the Gebel Elba National Park, without whom this project would not have been possible; the Conservation Leadership Programme for their continuous support; M. Fouda, Director of Nature Conservation Sector, and the Gebel Elba National Park staff for their support and assistance; Mohammed Gad, Mahmoud Hanafy, Samy Zalat, and Adam Saadallah and his staff for their special assistance to the project team; the LIFE Red Sea Project and the Center for Documentation of Cultural and Natural Heritage, in particular the Director, Hala Barakat, for their cooperation; Shaheer Youstos, Cloudia Yostso, and the Off Road Egypt Company for their support; and Mike Maunder and Pete Lowry for their constructive comments on the article.
Biographical sketches
Mohamed Kamel Ahmed's research interests include plant community and vegetation analysis, and the eco-physiological response to drought and salinity of the native plants in arid regions. Usama Mohammed Ghazaly has experience in the monitoring and conservation of plant diversity at Gebel Elba National Park, as well as in community work in remote rural areas of Egypt. Martin Callmander is interested in the systematics of various plant families and plant biogeography. He has considerable experience in IUCN Red Listing and in using botanical data in support of conservation in biodiversity hotspots.






