Introduction
Nature-based tourism is becoming more popular and is growing at a faster rate than more conventional forms of tourism (Newsome et al., Reference Newsome, Moore and Kingston2012), particularly in biodiversity-rich developing countries (Balmford et al., Reference Balmford, Beresford, Green, Naidoo, Walpole and Manica2009). In protected areas, tourism may improve conservation effectiveness by providing funds for management, research and education programmes (Newsome et al., Reference Newsome, Moore and Kingston2012; Leung et al., Reference Leung, Spenceley, Hvenegaard and Buckley2018). Furthermore, nature-based tourism is usually concentrated in a relatively small area and has more limited impacts than other economic activities such as logging and agriculture (Turton & Stork, Reference Turton and Stork2008). However, the effectiveness of tourism as a conservation-supporting strategy remains debatable (Das & Chatterjee, Reference Das and Chatterjee2015; Brandt & Buckley, Reference Brandt and Buckley2018). A global review revealed that negative effects of tourism on wildlife are relatively common (59% of the 274 studies) and that there is a major research gap on the impacts of tourism in the biodiversity-rich areas where ecotourism is expanding (Larson et al., Reference Larson, Reed, Merenlender and Crooks2016).
Negative impacts of tourism on wildlife are more likely in protected areas that harbour many species sensitive to human disturbance. In these areas, a constant human presence could drive changes in the use of space by species or in their temporal activity (Zhou et al., Reference Zhou, Buesching, Newman, Kaneko, Xie and Macdonald2013; Fortin et al., Reference Fortin, Rode, Hilderbrand, Wilder, Farley, Jorgensen and Marcot2016; Coppes et al., Reference Coppes, Burghardt, Hagen, Suchant and Braunisch2017). For instance, leopards were more active during the daytime and used tourist areas more frequently when a national park in Thailand was closed to visitors (Ngoprasert et al., Reference Ngoprasert, Lynam and Gale2017). Similarly, avoidance of areas near intensively used tourist trails caused indirect habitat loss for wolves and elks in Canada (Rogala et al., Reference Rogala, Hebblewhite, Whittington, White, Coleshill and Musiani2011). In some cases, even low-impact tourism can cause changes in species distributions and habitat use (Reed & Merenlender, Reference Reed and Merenlender2008; Fortin et al., Reference Fortin, Rode, Hilderbrand, Wilder, Farley, Jorgensen and Marcot2016). However, there are also situations in which wildlife does not seem to be affected by tourism (Blake et al., Reference Blake, Mosquera, Loiselle, Romo and Swing2017; Larm et al., Reference Larm, Erlandsson, Norén and Angerbjörn2019). In a large assessment of North American parks, habitat features outperformed tourism in explaining the distribution and use of space of mammal species (Kays et al., Reference Kays, Parsons, Baker, Kalies, Forrester and Costello2017).
Adequate management of tourism activity is essential in protected areas given that overcrowding and poor planning could result in the deterioration of biodiversity and scenic values. These negative impacts could compromise the conservation goals of protected areas and the ecosystem services they provide, including tourism (Turton & Stork, Reference Turton and Stork2008; Leung et al., Reference Leung, Spenceley, Hvenegaard and Buckley2018). Therefore, biodiversity monitoring programmes should be a priority in protected areas that have been opened for visitors, particularly where important biodiversity values overlap with high tourism potential. Such monitoring can serve as an early warning system for the need to change management schemes to promote the long-term maintenance of species (Yoccoz et al., Reference Yoccoz, Nichols and Boulinier2001). However, tourism-driven impacts are difficult to measure (Buckley, Reference Buckley2003) and the lack of data collected before the intensification or beginning of tourism makes these assessments even more challenging (Butsic et al., Reference Butsic, Lewis, Radeloff, Baumann and Kuemmerle2017). This is the case for national parks in Brazil, where tourism has been growing at an annual rate of 10% (ICMBio, Reference ICMBio2019) but studies assessing the impacts of visitors on biodiversity remain scarce (Cunha, Reference Cunha2010; Silva et al., Reference Silva, Paviolo, Tambosi and Pardini2018; Monteiro & Lira, Reference Monteiro and Lira2020).
Here we used a quasi-experimental setting to investigate the potential impacts of carefully planned nature-based tourism on mammal species at Cavernas do Peruaçu National Park, a high-priority area for conservation in Brazil (Ministério do Meio Ambiente, 2018). We surveyed the mammal community using camera traps on tourist and non-tourist trails before and after the Park officially opened for visitors. To our knowledge this is the first study of this type in a Brazilian national park using baseline data collected before the intensification of tourism. According to the risk–disturbance hypothesis (Frid & Dill, Reference Frid and Dill2002) and previous assessments conducted elsewhere (Rogala et al., Reference Rogala, Hebblewhite, Whittington, White, Coleshill and Musiani2011; Zhou et al., Reference Zhou, Buesching, Newman, Kaneko, Xie and Macdonald2013), we expected that some species would avoid or limit their use of tourist trails after visitors were allowed into the Park, causing a decline in species richness and their probability of trail use. Given that anthropogenic pressure can also modify the activity patterns of species (Marchand et al., Reference Marchand, Garel, Bourgoin, Dubray, Maillard and Loison2014; Gaynor et al., Reference Gaynor, Hojnowski, Carter and Brashares2018) we anticipated that the impacts of visitors could also lead to the temporal displacement of mammals. Notably, we expected that species would be less active and would show reduced diurnal activity on tourist trails after the beginning of tourism. Our intent with this assessment is not to jeopardize tourism but to inform effective management strategies that facilitate both biodiversity conservation and the development of low-impact tourism in the region.
Study area
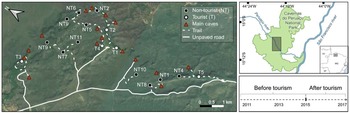
This study was conducted in Cavernas do Peruaçu National Park (Fig. 1), in south-eastern Brazil in the ecotone between Cerrado (Neotropical savannah) and Caatinga (a mosaic of thorn scrub and seasonally dry forests associated with a semi-arid climate; Leal et al., Reference Leal, Da Silva, Tabarelli and Lacher2005). The 568 km2 Park protects extensive areas of dry forests and woody savannah and supports 70% of all large mammals found in the Brazilian Cerrado (Ferreira & Oliveira, Reference Ferreira and Oliveira2014). The Peruaçu River is the main source of water in the Park and its valley harbours a unique speleological system with hundreds of caves and archaeological sites with major tourism potential. Gallery forests along the river and dry forests are the main vegetation types in the river valley (Oliveira-Filho & Ratter, Reference Oliveira-Filho and Ratter2002). The climate is semi-arid, with a mean annual temperature of 24.4 °C and a total mean annual rainfall of 925 mm concentrated in the wet season (mid October–March; Geoclock, Reference Geoclock2005).

Fig. 1 Study area and locations of the camera traps (dots) deployed to survey tourist and non-tourist trails in Cavernas do Peruaçu National Park, Brazil. A tourism timeline is represented in the bottom right.
Given that caves are the main tourist attraction in the Park and that these are also extremely fragile ecosystems, the potential negative impacts from tourist activity have long been a concern for those managing the Park. As such, a carefully designed plan for tourism was included in the Park's management plan (Geoclock, Reference Geoclock2005). Tourist visitation is restricted to the Peruaçu River valley in the central region of the Park and consists of guided visits to caves and rock art panels, which are accessed via dedicated trails. Before their visit, tourists must hire a certified local tour guide. They are then registered and informed about the rules in the Park, particularly restrictions on accessing non-tourist areas and walking off-trail. Each guide can host eight visitors at a time and there is a daily limit on the number of visitors allowed on each tourist route. The Park remained closed to tourism until roads, walkways, visitor centres, and other tourist infrastructure were improved or built, but a small number of visitors (200–600 per year) were allowed on a few tourist trails and caves during a pre-opening pilot scheme. The Park officially opened to tourists in 2015 (Fig. 1) and visitation increased substantially, reaching almost 7,000 tourists in 2017 (Supplementary Table 1).
Methods
Camera-trap surveys
To investigate the potential effects of visitors on the mammal community in the Park, we set passive infrared camera traps (Bushnell Trophycam, Bushnell Corporation, Overland Park, USA) at 16 sampling sites on pre-existing trails in tourist and non-tourist areas (mean minimum distance to the nearest sampling site was c. 0.77 km; Fig. 1, Supplementary Table 2). We conducted surveys during 2011–2017 restricted to the Peruaçu River valley, where all tourist routes are located (Table 1). At each site we deployed camera traps 1–2 m from the trail at a height of c. 30 cm, parallel to the ground and aimed at the trail. We set the camera traps to work continuously and record 10-s videos when triggered, with 30-s intervals between triggers. We removed thin vegetation directly in front of the cameras to prevent false triggers. We conducted maintenance to replace SD cards and batteries and to clear vegetation at 45–60 day intervals. We did not use baits or lures to attract animals.
Table 1 Details of the camera-trap surveys conducted in Cavernas do Peruaçu National Park, Brazil (Fig. 1).

Data analysis
We used four metrics derived from camera-trap data to assess the potential impacts of tourist visitation on the mammal community in the Park: species richness, probability of using trails, overall activity level and daily activity pattern. We based these metrics on records of medium- and large-sized mammal species > 1.0 kg and included one smaller rodent, the rock cavy Kerodon rupestris, which is reliably identifiable in camera-trap records. We classified camera-trap data according to visitation period: 2011–2014 as before tourism and 2015–2017 as after tourism. We assumed that the incipient tourism activity before the Park officially opened to tourism would have a negligible or much weaker impact than after official visitation started and the number of visitors increased substantially. Finally, we classified the trails where camera traps were deployed as tourist (n = 5) or non-tourist (n = 11; Supplementary Table 2). The unequal number of sites in each trail category was because of the relatively small area where tourism takes place in the Park, which would not support a larger number of camera-trap sites unless we reduced substantially the distance between neighbouring sampling sites.
We constructed a daily record history for each mammal species by assigning presence (1) at each camera-trap site where the species was recorded in a survey day (0.00–23.59) or absence (0) otherwise. Thus, one or more records of a species at a site within 24 h were considered as one independent record. We compared species richness using rarefaction curves and a jackknife 1 estimator with CI values for each camera-trap site before and after tourism under comparable sampling effort (i.e. number of camera-trap days; Colwell et al., Reference Colwell, Chao, Gotelli, Lin, Mao, Chazdon and Longino2012). Jackknife 1 is a non-parametric and incidence-based estimator that performs well with camera-trap datasets (Tobler et al., Reference Tobler, Carrillo-Percastegui, Leite Pitman, Mares and Powell2008).
We used binomial generalized linear mixed models to estimate the effects of tourism on the probability of trail use by six species, each with at least 100 independent records (Supplementary Table 3): ocelot Leopardus pardalis, paca Cuniculus paca, rock cavy, collared peccary (hereafter peccary) Pecari tajacu, grey brocket deer (hereafter deer) Mazama gouazoubira and coati Nasua nasua. We did not include tapeti Sylvilagus brasiliensis, with 128 independent records, in our assessment because models for the species did not converge. The relatively high number of records used as inclusion criteria was necessary for the convergence of models estimating up to seven parameters. The six target species are known to use trails in the Park and encompass a broad range of body sizes, feeding ecologies and behaviours, representing distinct natural history strategies of the local community of medium-sized and large mammals.
We used both visitation period and trail category as variables representing tourism in our models. We included the interaction between these factors as we anticipated that any potential responses to visitation period would be stronger on tourist trails. We also included vegetation type and seasonality as covariates because of their potential influence on probability of trail use by the target species, and we included camera-trap site as a random factor (Supplementary Table 4). Because our main objective was to assess the effects of tourism, we built alternative models that varied in their inclusion of vegetation type and seasonality covariates but holding tourism-related variables fixed (including their interactions). We used the Akaike information criterion with a correction for small sample sizes (AICc) to assess model support (Burnham & Anderson, Reference Burnham and Anderson2002).
We present the results for only the best-supported model for each species, as the effect of tourism-related variables in other concurrent models with ΔAICc < 6 did not change (Supplementary Tables 5 & 6). We followed standard procedures to assess model fit (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009; Hartig, Reference Hartig2020) by plotting standardized residuals vs model predictions as well as observed vs expected distribution of residuals, which indicated adequate model fit for all species (Supplementary Figs 1 & 2). We repeated the modelling procedures described above using a subset of the data to estimate the effect of visitors on the probability of trail use between 9.00 and 17.00, representing the core visitation hours when tourists are allowed in the Park. We conducted this additional analysis for five of the six target species, as we recorded pacas only rarely during the daytime.
The generalized linear mixed models implemented here do not account for any potential variation in detection probability. Statistical adjustments for imperfect detection can improve monitoring programmes (Mackenzie et al., Reference Mackenzie, Nichols, Lachman, Droege, Andrew and Langtimm2002) but the covariates influencing the detection probability can also be controlled prior to data collection through careful planning of the survey design (Banks-Leite et al., Reference Banks-Leite, Pardini, Boscolo, Cassano, Püttker, Barros and Barlow2014). Although adequate survey design might not fully eliminate imperfect detection, it can minimize variation in the detection probability that would affect the results. In our design, two features limited variation in the detection probability between sampling sites and survey periods: (1) we surveyed only pre-existing trails, avoiding the variation in detection between on- and off-trail sites, which is known to affect mammals in the region (Ferreira et al., Reference Ferreira, Ahumada, Oliveira, de Pinho, Barata, Carbone and Collen2017), and (2) at each site, camera traps were always deployed in the same tree, at the same height and facing the same direction during every survey, limiting the spatial and deployment effects on detection probability. Furthermore, we do not claim that a change in probability of trail use is driven necessarily by a change in animal abundance; instead, we interpret this as a metric reflecting the intensity of trail use by the species assessed, an approach that has been adopted in similar studies (Muhly et al., Reference Muhly, Semeniuk, Massolo, Hickman and Musiani2011; Blake et al., Reference Blake, Mosquera, Loiselle, Romo and Swing2017; Kays et al., Reference Kays, Parsons, Baker, Kalies, Forrester and Costello2017; Ngoprasert et al., Reference Ngoprasert, Lynam and Gale2017).
Finally, we investigated the effect of tourism on the activity of ocelots and rock cavies. We selected these species because they were amongst the most recorded species and were active during the daytime, and were thus more likely to be affected by visitors. To assess the effects of tourism on activity, we used all camera-trap records obtained for both species, not only the independent records. We estimated overall activity levels (proportion of time active) by fitting a flexible circular kernel distribution to time-of-detection data and we performed a Wald test to investigate whether the estimates before and after tourism differed significantly. Additionally, we conducted a Watson's two-sample test to compare the activity patterns of these species before and after tourism was allowed (Jammalamadaka & SenGupta, Reference Jammalamadaka and SenGupta2001; Oliveira-Santos et al., Reference Oliveira-Santos, Zucco and Agostinelli2013). To limit the potential effects of spatial variation on activity patterns, we conducted these pairwise comparisons independently for tourist and non-tourist trails. Similarly, to avoid the influence of vegetation type on activity, we restricted the comparisons to gallery forest sites, where we installed more survey sites on tourist trails. Analyses were conducted in R 3.6.3 (R Core Team, Reference R Core Team2020) using packages activity (Rowcliffe et al., Reference Rowcliffe, Kays, Kranstauber, Carbone and Jansen2014), overlap (Meredith & Ridout, Reference Meredith and Ridout2014) and circular (Agostinelli & Lund, Reference Agostinelli and Lund2017); we also used packages lme4 and MuMIn (Bates et al., Reference Bates, Maechler, Bolker and Walker2015; Barton, Reference Barton2016) for modelling and DHARMa (Hartig, Reference Hartig2020) for model checking. We estimated species richness with EstimateS 9.1.0 (Colwell, Reference Colwell2013).
Results
Species richness
We obtained 3,220 independent records of 23 mammal species (Supplementary Table 3). Estimated species richness varied between sites but within-site variation before and after the Park was opened for tourism was only moderate (Fig. 2). These variations in species richness were observed in both trail categories, although downwards trends were more frequent on tourist trails. However, none of this variation in species richness was statistically significant, with substantial overlap in the 95% CIs of the estimates for all within-site comparisons (Supplementary Table 7).

Fig. 2 Estimates of species richness (jackknife 1) in Cavernas do Peruaçu National Park (Fig. 1) before and after tourism was allowed at each survey site (connected by lines). The 95% CIs of the estimates for all pairwise comparisons overlapped, indicating that changes in species richness were not statistically significant (CIs not shown for presentation purposes; Supplementary Table 7).
Probability of trail use
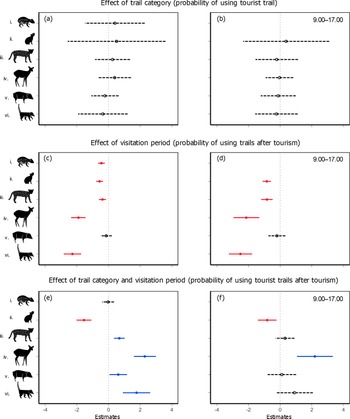
Trail category alone did not influence the probability of using trails by any of the target species across the whole study period (Fig. 3a). However, except for peccaries, all species showed reduced probabilities of using any trail after tourists were allowed in the Park (Fig. 3c), suggesting an overall decline in trail use during our study period. A more complex pattern emerged when we accounted for the interaction between trail category and tourist visitation. Contrary to our expectation, ocelots, deer, peccaries and coatis demonstrated higher probabilities of using tourist trails after tourism was allowed and this probability remained stable for pacas (Fig. 3e), indicating that the use of space by these species was not affected negatively by tourism. The rock cavy was the only species that responded as we expected, demonstrating a lower probability of using tourist trails after the intensification of tourism (Fig. 3e).

Fig. 3 Effects of tourism-related variables on the probability of trail use by six mammal species in Cavernas do Peruaçu National Park (model estimates displayed on a logit scale). (a,b) Effect of trail category (probability of using tourist trail at any period either before or after tourism was allowed), (c,d) effect of visitation period (probability of using any trail after tourism was allowed), and (e,f) effect of the interaction between trail category and visitation period (probability of using tourist trails after tourism was allowed). Right column (b,d,f) indicates the results when restricting the analysis to the core visitation hours (9.00–17.00). The generalized linear mixed model estimates (circles) and their 95% CIs (horizontal lines) are from the best-supported model for each species according to AICc values (Supplementary Tables 5 & 6). Species: (i) paca Cuniculus paca, (ii) rock cavy Kerodon rupestris, (iii) ocelot Leopardus pardalis, (iv) deer Mazama gouazoubira, (v) collared peccary Pecari tajacu and (vi) coati Nasua nasua. Filled circle and solid line: significant effect; Hollow circle and dashed line: non-significant effect.
When considering only the core visitation hours (9.00–17.00), we observed similar patterns for the effects of trail category (tourist vs non-tourist trails; Fig. 3b) and visitation (before vs after tourism was allowed; Fig. 3d). However, for peccaries, ocelots and coatis the observed increases in tourist trail use after the beginning of visitation disappeared when considering only the core visitation hours (Fig. 3f). By contrast, deer and rock cavies maintained the same responses as in the 24-h dataset, with the former showing an increased probability and the latter a decreased probability of tourist trail use after the intensification of tourism (Fig. 3f).
Activity parameters
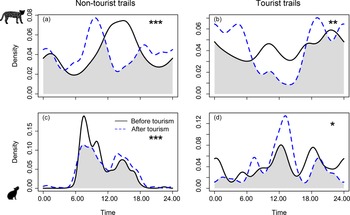
Tourism did not have a significant effect on overall activity levels (proportion of time species were active) for ocelots but it did influence the activity levels of rock cavies (Table 2). After tourism intensification, rock cavies had reduced overall activity on tourist trails and increased overall activity on non-tourist trails (Table 2). Both species altered their activity patterns (when species are active) following the beginning of tourist visitation in the Park (Fig. 4). For ocelots there were clear and significant changes in activity patterns in both trail categories (Fig. 4a,b), with a particularly strong decline for diurnal activity on tourist trails (Fig. 4b). On non-tourist trails, ocelots were largely diurnal both before and after tourism but their activity peak shifted from c. 14.00 to 10.00 after visitors were allowed in the Park (Fig. 4a). For rock cavies, virtually all of their activity on non-tourist trails was restricted to the daytime both before and after tourism but with a reduction in the morning peak (c. 7.30) after tourism was allowed (Fig. 4c). On tourist trails, contrary to our predictions, rock cavies showed increased diurnal activity after tourism was allowed, with a strong peak at 12.00 (Fig. 4d).

Fig. 4 Comparison of daily activity patterns, calculated using kernel density estimates, for ocelots (top row) and rock cavies (bottom row) before and after tourism was allowed in Cavernas do Peruaçu National Park (Table 2). Asterisks indicate significance levels resulting from Watson's two-sample test: *P < 0.05, **P < 0.01, ***P < 0.001.
Table 2 Influence of tourism on estimates of overall activity levels for the ocelot Leopardus pardalis and rock cavy Kerodon rupestris in Cavernas do Peruaçu National Park (Fig. 4).

Discussion
Spatial and temporal responses to tourism
Our results suggest that the initial years of tourism activity in Cavernas do Peruaçu National Park had only a modest impact on the local mammal community. We observed temporal responses by ocelots and rock cavies but limited negative spatial responses in most species. There is no evidence that visitors had an impact on species richness, and the probability of using a tourist trail after tourism was allowed either increased or remained stable for five of the six species assessed. If visitors were causing species avoidance, we would expect this impact to be stronger on tourist trails (Rogala et al., Reference Rogala, Hebblewhite, Whittington, White, Coleshill and Musiani2011; Zhou et al., Reference Zhou, Buesching, Newman, Kaneko, Xie and Macdonald2013), which was not the case. Although we observed a general decline in the probability of trail use by most of the target species after visitors were allowed in the Park, our results do not indicate that tourism was the main factor driving this decline or that it caused indirect habitat loss, except for rock cavies. We believe that other factors not related to tourism could be influencing the study system, such as a reduction in water availability in the Peruaçu River, but this hypothesis would need to be investigated before any inferences could be drawn.
Displacement of wildlife from tourist to non-tourist areas has been reported as a strategy employed by wild species to avoid human presence (Rogala et al., Reference Rogala, Hebblewhite, Whittington, White, Coleshill and Musiani2011; Morrison et al., Reference Morrison, Boyce, Nielsen and Bacon2014; Fortin et al., Reference Fortin, Rode, Hilderbrand, Wilder, Farley, Jorgensen and Marcot2016). We observed this only for rock cavies in our study. This nationally threatened rodent (ICMBio, Reference ICMBio2018) showed spatial and temporal avoidance of tourist areas after the intensification of tourism, as it reduced the use of tourist trails and increased activity levels on non-tourist trails, which indicates the species was affected by visitation. However, rock cavies showed increased diurnal activity on tourist trails after tourism, which contradicts our predictions of greater nocturnality to minimize interactions with humans. This complex response pattern needs further investigation and could be affected by interactions with predators as the increased diurnal activity of rock cavies was concurrent with the shift towards more nocturnal activity on tourist trails of ocelots. Rock cavies are diurnal (Portella & Vieira, Reference Portella and Vieira2016) and poached heavily for their meat (ICMBio, Reference ICMBio2018), which could explain their responses to the increased human presence. However, this rock-dwelling rodent can climb steep rock outcrops, thus our results do not necessarily indicate a complete displacement from tourist areas. To avoid human contact, rock cavies could be responding by exploring the vertical dimension of their habitat, therefore reducing detection.
Despite the negative response of rock cavies to visitors, four of the six target species showed increased probabilities of using a tourist trail after the Park was opened to tourists. Given that the implementation of tourism in the study area has followed high standards and visitors do not leave food or litter behind, we did not expect an increase in the use of tourist trails by any of the target species, particularly peccaries and deer, as Neotropical ungulates are often sensitive to human presence and affected by even low-intensity tourism (Blake et al., Reference Blake, Mosquera, Loiselle, Romo and Swing2017; Silva et al., Reference Silva, Paviolo, Tambosi and Pardini2018). In addition, peccary occupancy is known to be influenced negatively by anthropogenic pressure in the study area (Ferreira, Reference Ferreira2018). However, it is not uncommon for ungulates to show habituation to tourists (Stankowich, Reference Stankowich2008). It is possible that visitation could have created a zone in which the risk of poaching is lower, benefitting some species. Given that some level of poaching is known to occur in the Park (D. Barcelos & G.B. Ferreira, pers. obs., 2006, 2012, 2014, 2015), the unintentional patrolling of guides and visitors could have caused a reduction in this illegal activity in tourist areas.
Although four species showed increased use of tourist trails after the intensification of tourism, this occurred outside the core visitation hours (9.00–17.00) for ocelots, peccaries and coatis. This suggests a nuanced response to visitors in which these species increased their use of tourist trails to benefit from changes caused by tourism (e.g. refuge from predators or poachers) while still limiting their direct interactions with people. Shifts towards more nocturnal activity in tourist areas have been reported elsewhere (Marchand et al., Reference Marchand, Garel, Bourgoin, Dubray, Maillard and Loison2014; Coppes et al., Reference Coppes, Burghardt, Hagen, Suchant and Braunisch2017) and we detected a similar shift in the activity patterns of ocelots in this study. We found unusually high diurnal activity for ocelots, not reported for the species elsewhere (Maffei et al., Reference Maffei, Noss, Cuéllar and Rumiz2005; Di Bitetti et al., Reference Di Bitetti, Paviolo and De Angelo2006; Kolowski & Alonso, Reference Kolowski and Alonso2010), which shifted to nocturnal activity on tourist trails after visitors were allowed into the Park.
Implications for the management of tourism activity
Our results suggest that tourism management strategies such as those adopted at Cavernas do Peruaçu National Park (e.g. zoning, a dedicated trail system, a daily cap of visitors and a requirement for certified tour guides) may limit wildlife displacement from tourist areas. However, we noticed some responses to tourism that were particularly strong and potentially detrimental to the nationally threatened rock cavy. Considering that even quiet, non-consumptive tourism can cause negative impacts on species (Reed & Merenlender, Reference Reed and Merenlender2008) and that most mammals are likely to respond to people to some degree (Larson et al., Reference Larson, Reed, Merenlender and Crooks2016; Gaynor et al., Reference Gaynor, Hojnowski, Carter and Brashares2018), zero-impact tourism activity may be unachievable and should not be a target of tourism management programmes in protected areas. Therefore, if some level of impact is likely to occur, a realistic management strategy should address two distinct features of such impact: spatial distribution and intensity.
Zoning is essential to keep negative impacts from tourism as localized as possible and to avoid compromising the conservation objectives of protected areas (Leung et al., Reference Leung, Spenceley, Hvenegaard and Buckley2018). Limiting the tourist area ensures that eventual negative impacts will be limited only to a proportion of the animal populations protected in the region. Additionally, a sensible cap in the daily number of tourists (as is the current practice in our study area) is likely to limit the intensity of these impacts. The number of visitors is known to modulate the impacts of tourism on local biodiversity (Das & Chatterjee, Reference Das and Chatterjee2015) and wildlife avoidance of tourist areas has been reported in highly visited Brazilian national parks (Cunha, Reference Cunha2010; Silva et al., Reference Silva, Paviolo, Tambosi and Pardini2018; Monteiro & Lira, Reference Monteiro and Lira2020). Given that tourism activity has only recently begun and has been growing substantially in the Park (Supplementary Table 1), it is important to realize that the effects of a larger number of tourists could be different from what has been observed in the initial years of visitation, and constant monitoring is necessary to assess any medium- to long-term effects.
The rock cavy was the only species that showed a negative spatial response to the beginning of visitation. This is of particular concern for a nationally threatened species as displacement from some areas of the Park would reduce the habitat effectively available for the population. Because the Park is one of the few protected areas where rock cavies occur in Minas Gerais state, it is paramount to mitigate any negative impacts on this population. To this end, understanding the mechanisms driving rock cavy responses to the intensification in tourism should be a priority so that effective management strategies can be adopted. Furthermore, any decline in the local rock cavy population would be detrimental for the tourism sector as this is the only native mammal species regularly observed in the Park, therefore improving visitor experience.
Our assessment had the limitation of monitoring only trails leading to caves and not caves in particular, which are the main tourist attractions in the region. Nonetheless, these fragile environments provide crucial habitats for bats and invertebrates (Ferreira & Horta, Reference Ferreira and Horta2001; Paksuz & Özkan, Reference Paksuz and Özkan2012; de Sousa Barros et al., Reference de Sousa Barros, Bernard and Ferreira2021); in our study area they support a high diversity of troglobites (Trajano et al., Reference Trajano, Gallão and Bichuette2016) and are used frequently by Neotropical otters Lontra longicaudis (Pinho et al., Reference Pinho, Ferreira and Barata2018). Our study does not allow us to draw any inferences regarding the impacts of visitors on species restricted to or highly associated with caves and a specific monitoring scheme is needed to examine this.
Taken together, our results suggest that the mammal community and most of our target species were able to tolerate visitation during the initial years of tourism activity in the Peruaçu River valley without being displaced from tourist areas. However, because time lags between impacts and responses of species are common in natural systems (Watts et al., Reference Watts, Whytock, Park, Fuentes-Montemayor, Macgregor, Duffield and McGowan2020), our findings should be viewed with caution as they correspond only to the initial phase of tourism in the Park. Furthermore, the tourism management interventions adopted probably worked in tandem with the low numbers of tourists visiting the Park compared to better-known Brazilian national parks (ICMBio, Reference ICMBio2019). Therefore, we suggest that a multi-taxa and robust monitoring system measuring biodiversity responses to tourism should be implemented to inform an adaptive management programme as tourism activity develops further. This would allow managers to make and adapt decisions based on ecological knowledge, thereby increasing the probability of conservation goals being achieved (Leung et al., Reference Leung, Spenceley, Hvenegaard and Buckley2018).
Considering, however, that some degree of change caused by tourism may be inevitable, it is also important to agree on what level of impact would be acceptable in a protected area. This complex issue should not be addressed by ecologists alone and the engagement of other stakeholders in establishing this limit is essential for setting sensible targets. Moreover, any negative impacts on biodiversity caused by visitors should be weighed against the conservation and management gains provided by tourism. In our study area, organized tourism has, directly or indirectly, brought increased funding, improved infrastructure, greater recognition and unintentional patrolling to the Park, which together have probably improved conservation effectiveness. Additionally, tourism is generating employment and income for local communities, thereby improving their perceptions of the Park and potentially reducing any conflicts that could adversely affect biodiversity. These benefits and the results presented here support the possibility of accommodating nature-based tourism and effective biodiversity conservation at Cavernas do Peruaçu National Park.
Acknowledgements
We thank I.M. Barata, F.F. Pinho, M.J.R. Oliveira, L. Bonjorne and many others for field assistance; B.E. Lopes and C.R. Córdova for assistance with data management; park managers and employees of Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), particularly Norivaldo Pereira dos Santos, for their support; and the Conservation Leadership Programme, Panthera, Idea Wild, International Foundation for Science (IFS) (5353-1), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)/Programa de Pesquisa em Biodiversidade (PPBio)/Rede ComCerrado (457434/2012-0), Centro Nacional de Pesquisas e Conservação de Mamíferos Carnívoros (CENAP)/ICMBio and WWF-Brasil (190-2012) for research funding. DCB received a scholarship from CNPq (131032/2016-0) and a research grant from Decanato de Pesquisa e Pós-Graduação (DPP)/Universidade de Brasília (UnB). EMV received a personal research grant from CNPq (311988/2017-2).
Author contributions
Study design: GBF; data collection: GBF, DCB, MSP; data analysis: DCB, GBF; writing: DCB, GBF, EMV.
Conflicts of interest
DCB, MSP and GBF represented Instituto Biotrópicos on the Cavernas do Peruaçu National Park advisory council during 2014–2016.
Ethical standards
This research abided by the Oryx guidelines on ethical standards.