Introduction
Globally, human–wildlife conflict is an increasing threat to species conservation. Wildlife conflicts include crop damage, livestock depredation and human injury (Naughton-Treves, Reference Naughton-Treves1998; Madden, Reference Madden2004; Acharya et al., Reference Acharya, Paudel, Neupane and Kohl2016; Nyhus, Reference Nyhus2016). With climate warming-driven shifts in resource availability and species distributions there is an increased potential for human–wildlife conflict (LeDee et al., Reference LeDee, Handler, Hoving, Swanston and Zuckerberg2020). However, addressing such conflict is a complex process that involves many stakeholders with various perspectives and objectives. Therefore, the approaches taken to reduce conflicts differ widely (Dickman, Reference Dickman2010; White & Ward, Reference White and Ward2010).
Most conflicts with wildlife are associated with habitat loss or modification, changes in resource availability, increasing human populations, incursion into wildlife habitats and the provision of anthropogenic food that can supplement energy input (Nyhus, Reference Nyhus2016; Plaza & Lambertucci, Reference Plaza and Lambertucci2017). Supplemental food can have both positive and negative effects on wildlife, including changes in distribution, body condition, survival, reproduction and population dynamics (Lunn & Stirling, Reference Lunn and Stirling1985; Stringham, Reference Stringham and Bromley1989; Becker et al., Reference Becker, Streicker and Altizer2015; Lillie et al., Reference Lillie, Gese, Atwood and Conner2019). For example, polar bears feeding on subsistence-harvested bowhead whale Balaena mysticetus carcasses ingest low-fat and high-protein foods that could result in increases in energetic expenditures and lead to health problems (Rode et al., Reference Rode, Robbins, Stricker, Taras and Tollefson2021). Although anthropogenic food can benefit wildlife, it often brings animals near to humans and could result in conflict. Management is particularly challenging when access to anthropogenic food results in conflicts with carnivores because of the combination of both perceived and real threats to people and their property (Naughton-Treves et al., Reference Naughton-Treves, Grossberg and Treves2003; Treves & Karanth, Reference Treves and Karanth2003; Morehouse & Boyce, Reference Morehouse and Boyce2017).
Conflicts between people and bears have occurred wherever and whenever their distributions overlap (Rajpurohit & Krausman, Reference Rajpurohit and Krausman2000; Dai et al., Reference Dai, Hacker, Zhang, Li, Li, Xue and Li2020; Krofel et al., Reference Krofel, Elfström, Ambarlı, Bombieri, González-Bernardo, Jerina, Melletti and Penteriani2020) and often because of bears being attracted to anthropogenic foods (Lunn & Stirling, Reference Lunn and Stirling1985; Craighead et al., Reference Craighead, Summer and Mitchell1995). Unsecured food and food waste can lead food-conditioned bears to associate humans with food rewards (Herrero et al., Reference Herrero, Smith, DeBruyn, Gunther and Matt2005), resulting in property damage, human injury and death (Herrero, Reference Herrero2018).
The relationship between black bears Ursus americanus and brown bears Ursus arctos (including grizzly bears) and anthropogenic attractants is well documented in the continental USA (Wilson et al., Reference Wilson, Madel, Mattson, Graham, Burchfiled and Belsky2005; Mazur, Reference Mazur2010). When the US government established a national park and associated visitor facilities, dumps were built to handle waste. Bears then began foraging at these anthropogenic food sources (Craighead et al., Reference Craighead, Summer and Mitchell1995). As bears became food-conditioned, conflicts escalated, resulting in human injuries and property damage (Craighead et al., Reference Craighead, Summer and Mitchell1995; Mazur & Seher, Reference Mazur and Seher2008; Herrero, Reference Herrero2018). Attempts to control human–bear conflict were often conducted in the absence of an understanding of the underlying causal factors (Haroldson et al., Reference Haroldson, Schwartz and Gunther2008; Elfström et al., Reference Elfström, Davey, Zedrosser, Müller, De Barba and Støen2014). Although public education, hazing, relocation and waste control actions were implemented, abrupt dump closures resulted in increased conflicts as food-conditioned bears sought alternative sources of anthropogenic foods. In Yellowstone National Park, dump closures resulted in a marked increase in brown bear mortality and an associated population decline (Craighead et al., Reference Craighead, Summer and Mitchell1995; Haroldson et al., Reference Haroldson, Schwartz and Gunther2008). Once habituated, food-conditioned bears might damage property and injure people to obtain food (McCarthy & Seavoy, Reference McCarthy and Seavoy1994; Mazur & Seher, Reference Mazur and Seher2008; Elfström et al., Reference Elfström, Davey, Zedrosser, Müller, De Barba and Støen2014; Lillie et al., Reference Lillie, Gese, Atwood and Sonsthagen2018). Additional challenges arise from parent–offspring learning in bears that can result in anthropogenic food use in new generations (Lunn & Stirling, Reference Lunn and Stirling1985; Mazur & Seher, Reference Mazur and Seher2008). Ultimately, preventing bears from gaining access to anthropogenic food is necessary to reduce conflicts and associated bear mortalities.
Because of their low population density and dependence on remote sea-ice habitats, conflicts between people and polar bears Ursus maritimus have been less frequent than those between people and black and brown bears (Krofel et al., Reference Krofel, Elfström, Ambarlı, Bombieri, González-Bernardo, Jerina, Melletti and Penteriani2020). Although less studied, human–polar bear conflicts have a long association with anthropogenic foods (Lunn & Stirling, Reference Lunn and Stirling1985; Dyck, Reference Dyck2006; Rogers et al., Reference Rogers, Peacock, Simac, O'Dell and Welker2015). Polar bears on land are often food-deprived, and sea-ice loss driven by climate warming has lengthened the durations that polar bears spend on land (Castro de la Guardia et al., Reference Castro de la Guardia, Derocher, Myers, Terwisscha van Scheltinga and Lunn2013; Stern & Laidre, Reference Stern and Laidre2016). Longer ice-free seasons increase the energetic stresses on fasting polar bears (Castro de la Guardia et al., Reference Castro de la Guardia, Derocher, Myers, Terwisscha van Scheltinga and Lunn2013; Hamilton et al., Reference Hamilton, Castro de la Guardia, Derocher, Sahanatien, Tremblay and Huard2014; Molnár et al., Reference Molnár, Bitz, Holland, Kay, Penk and Amstrup2020) and can lead to more polar bears seeking food near human settlements (Stirling & Derocher, Reference Stirling and Derocher1993; Towns et al., Reference Towns, Derocher, Stirling, Lunn and Hedman2009; Heemskerk et al., Reference Heemskerk, Johnson, Hedman, Trim, Lunn, McGeachy and Derocher2020).
Here we examine case studies of polar bear attraction to anthropogenic food and its role as a source of human–bear conflict. We conclude with a consideration of the conservation and management implications of anthropogenic food reliance.
Case studies
Polar bears are distributed across the Arctic in 19 subpopulations within the jurisdictions of five countries (the USA, Canada, Greenland, Norway and Russia; IUCN/SSC Polar Bear Specialist Group, Reference Durner, Laidre and York2018) and are categorized as Vulnerable on the IUCN Red List (Wiig et al., Reference Wiig, Amstrup, Atwood, Laidre, Lunn and Obbard2015). We examine human–polar bear conflicts associated with anthropogenic food sources in six areas with a range of social and environmental conditions (Fig. 1).
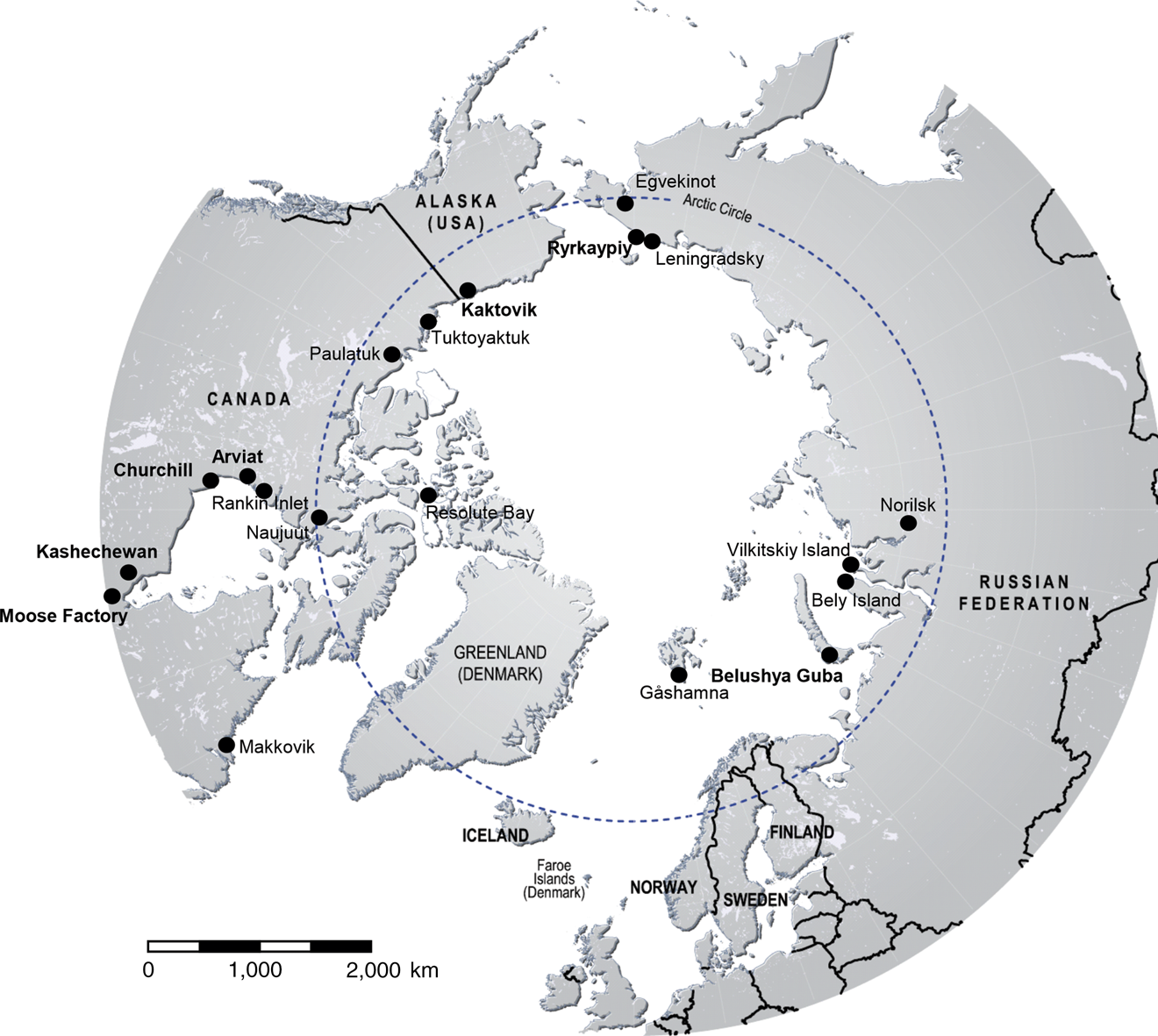
Fig. 1 Locations of polar bear visits to dumps (Table 1) to exploit anthropogenic food sources. The case study locations are indicated in bold.
Churchill, Manitoba, Canada
The western Hudson Bay polar bear subpopulation spends most of the year on the annual sea ice of Hudson Bay. When the ice melts in summer, the entire subpopulation is forced onto land. As sea ice reforms in autumn, polar bears migrate northward. This seasonal phenomenon brings them close to the town of Churchill (population c. 900 people) and often results in human–polar bear conflicts (Towns et al., Reference Towns, Derocher, Stirling, Lunn and Hedman2009).
Polar bear attraction and use of anthropogenic food increased when a military base and a beluga Delphinapterus leucas whaling station were established in 1942, increasing food attractants in and near Churchill (Stirling et al., Reference Stirling, Jonkel, Smith, Robertson and Cross1977). Use of a dump near the town was dominated by subadults and family groups (Lunn & Stirling, Reference Lunn and Stirling1985). By the 1960s food-conditioned polar bears at Churchill became a public safety concern as polar bears damaged property and inflicted injuries on people whilst in search of anthropogenic food beyond the dump (Fleck & Herrero, Reference Fleck and Herrero1988; Struzik, Reference Struzik2014). Although supplemental food from the dump was deemed to have minimal survival or reproductive benefit, polar bears returned to the dump habitually every year (Lunn & Stirling, Reference Lunn and Stirling1985). This study also noted that in Nunavut, the jurisdiction north of Manitoba, polar bears that used the Churchill dump were twice as likely to be hunted by Inuit, for subsistence, compared to polar bears that did not use the dump.
Government-sponsored programmes dedicated to dealing with Churchill's growing polar bear problem (Polar Bear Control Program in 1969 and Polar Bear Alert Program after 1984) were initiated to ensure human safety and the protection of property (Kearney, Reference Kearney and Bromley1989). Changes to polar bear management policies in the 1980s resulted in an effort to reduce the number of polar bears using the dump habitually, through hazing, relocation, temporary housing of polar bears at a holding facility until the Hudson Bay froze, transportation to zoos or other captive facilities, and lethal removal (Towns et al., Reference Towns, Derocher, Stirling, Lunn and Hedman2009; Struzik, Reference Struzik2014; Heemskerk et al., Reference Heemskerk, Johnson, Hedman, Trim, Lunn, McGeachy and Derocher2020). The Churchill town dump was closed in 2005 (Towns et al., Reference Towns, Derocher, Stirling, Lunn and Hedman2009) and there was no notable increase in the number of human–polar bear conflicts near Churchill. However, ascribing cause and effect to these various actions is not possible because of a concurrent decline in subpopulation abundance and changing management practices, such as dump closures (Heemskerk et al., Reference Heemskerk, Johnson, Hedman, Trim, Lunn, McGeachy and Derocher2020).
The Churchill experience with dump closure resulted in markedly different outcomes from those seen with the dump closures in Yellowstone National Park. In Churchill, no increase in human–polar bear conflicts occurred post-dump closure because a well-developed plan and infrastructure were in place to address such conflicts, the most problematic food-conditioned polar bears had been removed before dump closure and the subpopulation was declining in abundance because of climate warming. Closing the dump, securing waste within a polar bear-proof facility and public education decreased damage and human injury.
Arviat, Nunavut, Canada
Human–polar bear conflict has increased in recent years in some Nunavut communities. This has been attributed to increased human presence on the land and increased polar bear abundance; polar bears have spent more time onshore because of delayed sea-ice formation driven by climate warming (Dowsley & Wenzel, Reference Dowsley and Wenzel2008). Arviat is a coastal community of western Hudson Bay (population c. 3,000 people) in Nunavut, Canada. Polar bears in this area are part of the western Hudson Bay subpopulation, which declined from c. 1,200 to c. 800 polar bears during 1987–2011 (Lunn et al., Reference Lunn, Servanty, Regehr, Converse, Richardson and Stirling2016). Despite this decline in abundance, human–polar bear conflicts have increased over the past several decades.
Arviat has an open dump that provides anthropogenic food for polar bears. As a result, the number of polar bears using the dump has grown each year since record keeping began in the 1960s (Savikataaq, Reference Savikataaq2014). A deterrent programme instituted in 2010 has reduced the destruction of problem polar bears but the dump remains a significant attractant. Delayed sea-ice formation in Hudson Bay (Castro de la Guardia et al., Reference Castro de la Guardia, Myers, Derocher, Lunn and Terwisscha van Scheltinga2017) has led to reduced body condition of polar bears (Stirling et al., Reference Stirling, Lunn and Iacozza1999; Sciullo et al., Reference Sciullo, Thiemann and Lunn2016) and could be responsible for the increased use of the dump as polar bears migrate northwards along the western coast (Towns et al., Reference Towns, Derocher, Stirling, Lunn and Hedman2009). This case study illustrates how a subpopulation of polar bears that is declining in abundance because of the loss of sea-ice habitat could change movement patterns in response to human food waste, resulting in increased human–polar bear conflicts where few have occurred historically.
Belushya Guba, Novaya Zemlya, Russia
In February 2019, Novaya Zemlya, a remote Russian archipelago between the Barents Sea and the Kara Sea, experienced a ‘mass invasion’ of polar bears (TASS Russian News Agency, Reference TASS Russian News Agency2019). Reports indicated that 52 polar bears had been drawn to a dump near the village of Belushya Guba (population c. 2,000 people). For 2 weeks, dozens of polar bears congregated at the open dump. Some bears entered the village, attempting to gain access to homes and civic buildings. Consequently, local Russian authorities declared a state of emergency on 16 February 2019 that lasted 10 days (TASS Russian News Agency, Reference TASS Russian News Agency2019). Although polar bears had visited the dump in previous years, this event was unprecedented and likely the result of a lengthened on-shore fasting period because of the loss of sea ice in the region. From the perspective of human–polar bear conflict, the situation at Belushya Guba illustrates that changes in the level of polar bear use of anthropogenic food can occur rapidly. There is concern that it is probable large numbers of polar bears will return to the area in the future unless access to anthropogenic food sources is eliminated.
Ryrkaypiy, Chukotka, Russia
In early December 2019, 60 polar bears congregated at a dump near the village of Ryrkaypiy (population c. 600 people) in Russia's Chukotka region (Odynova, Reference Odynova2019). Ryrkaypiy is 0.8 km from the Chukchi Sea. The open dump is a few hundred metres from the village. Polar bears had unrestricted access to the dump and congregated there until sea-ice formation occurred in late autumn. Both young and old polar bears were observed at the dump, and all were reported to be of below-average body condition. Delayed sea-ice formation and poor body condition probably account for polar bear use of this dump (Odynova, Reference Odynova2019). These polar bears also entered the village, damaged property and threatened inhabitants. Consequently, all public events were cancelled, children were not allowed outside and polar bear monitors worked to keep the polar bears out of the village. Once the sea ice formed and thickened, polar bears returned to the Chukchi Sea. This case illustrates that sea-ice conditions and polar bear body condition are predictors of human–polar bear conflicts and, as in other areas, dumps are an attraction to polar bears and can increase the numbers of problem animals in settlements.
Kaktovik, Alaska, USA
Located on the North Slope of Alaska, Kaktovik is a predominantly Inupiat community (population c. 180 people), with a history of subsistence harvest of bowhead whales. The remains of butchered bowhead whale carcasses left on beaches where butchering has occurred are a rich food source for a portion of the Southern Beaufort Sea polar bear subpopulation, ranging from minor dietary contributions (Boucher et al., Reference Boucher, Derocher and Richardson2019) to 15% of their diet (Bourque et al., Reference Bourque, Atwood, Divoky, Stewart and McKinney2020). Approximately 16% of the Southern Beaufort Sea polar bear subpopulation visits whale carcasses annually at Kaktovik (Lillie et al., Reference Lillie, Gese, Atwood and Conner2019).
Polar bears that were once drawn to the now protected Kaktovik community dump are attracted by bowhead whale remains following hunter harvesting. As many as 90 polar bears drawn from up to 160 km away gather near Kaktovik each autumn to feed on whale carcasses. A similar situation occurs in Barrow, Alaska (Herreman & Peacock, Reference Herreman and Peacock2013), and this is probably related to increased land use by the Southern Beaufort Sea polar bear subpopulation and the availability of bowhead whale remains from subsistence hunts (Miller et al., Reference Miller, Schliebe and Proffitt2006). As in other examples where human-mediated food creates an attraction, whale remains represent a significant source of energy to polar bears, yet polar bears feeding on whale remains could suffer negative health effects (Rode et al., Reference Rode, Robbins, Stricker, Taras and Tollefson2021) and they often enter the community, damage property and threaten people. Persistent and potentially dangerous polar bears that have become accustomed to entering the community are sometimes euthanized because of public safety concerns. A growing concern is the development of polar bear viewing tourism in the town. Such tourism provides an economic incentive to retain whale carcasses near town, presenting management challenges. This case study illustrates the challenges facing northern communities where traditional harvesting activities interact with climate warming-driven shifts in polar bear distributions and with their increased reliance on anthropogenic food.
First Nations coastal communities in Ontario, Canada
The Southern Hudson Bay subpopulation of polar bears lives on the sea ice of south-eastern Hudson Bay and James Bay to the south. However, during the ice-free season these polar bears move onto islands and coastal areas of James Bay and the Hudson Bay coast of Ontario. First Nations communities (Fort Severn, Peawanuck, Kashechewan, Attawapiskat and Moosonee/Moose Factory, population c. 250–3,500 people in each) are located along the western coast of James Bay and near the Hudson Bay coast. Human–polar bear conflicts related to attractants occur periodically at seasonal camps and communities in these areas (Lemelin et al., Reference Lemelin, Dowsley, Walmark, Siebel, Bird and Hunter2010). Spring goose hunting, during which hunters live in small cabins or tent camps along the coast, often results in conflict as this is when bears may come off the ice and approach camps. On shore, polar bears raid caches of harvested geese and are subsequently killed to protect the harvest. In the late summer or autumn before sea ice forms, some polar bears visit dumps near these communities. Occasionally, polar bears are found south of the area that is typically occupied during the ice-free season (Obbard & Middel, Reference Obbard and Middel2012). In July 2016 an adult female polar bear and two yearlings were found in the Kashechewan community dump (Canadian Broadcasting Corporation, 2016). One of the yearlings was shot when the family group entered town. Farther south, in December 2015 a single polar bear was shot at the Moose Factory dump (2 km north of the community) in the interest of public safety (Canadian Broadcasting Corporation, 2015), and in December 2020 an adult female polar bear and two yearlings were translocated from the Moose Factory dump (Moose Cree First Nation, 2020).
Polar bear responses to small-scale attractants
The above case studies illustrate the relationship between polar bears and large-scale attractants in the form of unsecured dumps or supplemental food. Dumps affect polar bears by providing an abundant, spatially and temporally predictable source of nutrition that is often exploited during lengthy periods when natural food sources are not available. However, human–polar bear conflict has a long and often unrecorded history involving much smaller-scale, ephemeral attractants such as those at hunting camps in Ontario. Additional examples include camps supporting resource extraction (e.g. oil and gas), tourism and research activities. Even in communities with secure dumps, polar bears could be attracted to animal carcasses stored outside homes, cooking odours, other human-related scents and areas where dogs are yarded. The following cases highlight situations in which polar bears were attracted to small-scale attractants, with one resulting in two polar bears being killed and the other resulting in a human fatality along with two polar bear fatalities.
On 5 July 1998, near Gåshamna in Hornsund, Svalbard, a group of 17 ecotourists and researchers established a field camp. On 8 August 1998, a polar bear described as appearing hungry and aggressive was observed near the cooking tent used by the group. The bear approached to within 20 m of people and was shot and killed. On the following day a second polar bear was observed near the camp's rubbish burning site. When this bear rapidly approached two people, it was shot at a distance of 6–10 m. Attempts to deter these polar bears with noise makers and warning gunshots failed. A necropsy found rubbish in the stomach of one of the polar bears.
In August 2018, on White Island near the town of Naujaat, Nunavut, three subsistence hunters were approached by a female polar bear and her yearling at their camp (Rogers, Reference Rogers2018). The adult female attacked and killed one person before the others, who had been lightly injured, could kill both bears. These polar bears were reported to have been in good condition (Rogers, Reference Rogers2018). It is possible, but uncertain, that anthropogenic scents (e.g. food, harvested animals) at the camp could have attracted these polar bears.
These cases highlight that both human and polar bear fatalities have been, and probably will continue to be, associated with attractants at camps. With increasing tourism in the Arctic (Runge et al., Reference Runge, Daigle and Hausner2020), negative interactions with polar bears are likely to increase.
Discussion
Unsecured dumps and other sources of anthropogenic food can represent sources of nutrition for polar bears when climate warming-driven sea-ice loss makes natural food less available. Polar bears often remain at these sources until they are driven off, access is barred or natural food becomes available. We have shown that human conflicts with polar bears are often associated with attractants. Although human-inflicted mortality of bears drawn to anthropogenic wastes is a management concern, disease transmission rates are also a concern because bears congregate in close proximity to each other and to other scavenging species when drawn to dumps. Little is known about disease transmission rates amongst congregating bears, but transmission rates are elevated where bears congregate at anthropogenic food sources (Atwood et al., Reference Atwood, Duncan, Patyk, Nol, Rhyan and McCollum2017; Whiteman et al., Reference Whiteman, Harlow, Durner, Regehr, Amstrup and Ben-David2018). There are numerous examples of other species experiencing higher rates of disease transmission and outbreak when congregated at anthropogenic food sources (Sorensen et al., Reference Sorensen, van Beest and Brook2014).
Food obtained from dumps is a poor substitute for the bears' natural diet (Plaza & Lambertucci, Reference Plaza and Lambertucci2017; Rode et al., Reference Rode, Robbins, Stricker, Taras and Tollefson2021). Polar bears cannot subsist entirely on terrestrial food because it lacks adequate levels of fat (Rode et al., Reference Rode, Robbins, Nelson and Amstrup2015a), and although food ingested at dumps was found to increase polar bear body mass when compared to polar bears that did not use dumps, reproductive and survival rates did not increase (Lunn & Stirling, Reference Lunn and Stirling1985). As indiscriminate foragers, polar bears often consume non-food waste contaminated with food residues. This includes plastics (e.g. bread bags, nappies, food waste in rubbish bags), metals (e.g. aluminium foils with food residues, tin cans, batteries), wood (e.g. toothpicks, skewers) and other materials that either contain food (e.g. ceramics) or were coated with food. Instances of polar bears experiencing serious health issues or dying because of ingested materials at dumps have been reported (Lunn & Stirling, Reference Lunn and Stirling1985; Dickie, Reference Dickie2019; A.E. Derocher, pers. obs., 2020; R. Stimmelmayr, pers. obs., 2020).
Polar bears that feed on anthropogenic food have higher mortality rates than those that do not (Lunn & Stirling, Reference Lunn and Stirling1985; McCarthy & Seavoy, Reference McCarthy and Seavoy1994). Although not all food-conditioned bears attack humans, individuals that do attack are often food-conditioned (Herrero, Reference Herrero2018). When feeding on anthropogenic food, a link between human scent and anthropogenic food is established. Upon entering a camp and detecting a human scent, those same polar bears occasionally enter tents in search of the food they expect to be present (Herrero, Reference Herrero2018). Such polar bears are often shot either by campers or by the authorities. Translocation is generally not an option for food-conditioned bears because they often resume their search for anthropogenic food when released (Hopkins & Kalinowski, Reference Hopkins and Kalinowski2013). For these reasons, the management of anthropogenic scents (i.e. attractant security) should be a high priority throughout the range of polar bears.
In summary, we have presented the following concerns associated with the use of anthropogenic food by polar bears: (1) Various forms of anthropogenic food can exacerbate human–polar bear conflict by attracting polar bears to come into close contact with people. (2) If anthropogenic food becomes unavailable or is reduced, food-conditioned polar bears may seek alternative sources, leading them to investigate nearby settlements. (3) Polar bears consuming anthropogenic food may be exposed to harmful chemicals and pollutants, could ingest non-food items or could be exposed to novel diseases or parasites. (4) Feeding on anthropogenic food disrupts the natural movement and distribution of polar bears. (5) Polar bears seeking anthropogenic food may form unnatural aggregations, with negative consequences for both polar bears and people. (6) Variations in the size, temporal, and spatial dynamics of attractants necessitates diverse management strategies. (7) Affected communities are small and often lack the resources to manage anthropogenic attractants.
Problems of food conditioning in polar bears have been reported in all five polar bear range states (the USA, Russia, Canada, Greenland and Norway; Wilder et al., Reference Wilder, Vongraven, Atwood, Hansen, Jessen and Kochnev2017; Fig. 1, Table 1). As polar bears spend increasing amounts of time on land as a result of climate warming-driven sea-ice loss, the likelihood of them seeking anthropogenic food increases (Stirling & Derocher, Reference Stirling and Derocher1993; Derocher et al., Reference Derocher, Lunn and Stirling2004). Along with decreasing body reserves and reduced survival as a result of polar bears spending less time on ice (Molnár et al., Reference Molnár, Bitz, Holland, Kay, Penk and Amstrup2020), human–polar bear conflict may become an increasing source of polar bear mortality if not addressed. Unless anthropogenic sources of energy are secured, this could represent an increasing threat to the viability of some polar bear subpopulations and decrease the safety of people living in affected regions.
Table 1 Locations, type of food and dates of polar bears Ursus maritimus visiting dumps across the Arctic.

Although the overarching threat from climate warming-driven habitat loss will take many years to resolve, managing anthropogenic waste should be addressed now. Work with black and brown bears has shown that once access to anthropogenic food is eliminated, bears return to their natural feeding strategies (Baruch-Mordo et al., Reference Baruch-Mordo, Wilson, Lewis, Broderick, Mao and Breck2014; Lewis et al., Reference Lewis, Baruch-Mordo, Wilson, Breck, Mao and Broderick2015) and human–bear conflicts decrease.
We do not need innovative solutions to the problem of anthropogenic waste attracting polar bears, but rather an immediate and concerted effort throughout the Arctic to apply what is already known. Education, the implementation of polar bear-proof methods of waste storage, law enforcement and the provision of adequate resources at the community level are required to mitigate this potentially increasing problem. All communities noted here were small, and thus have limited resources to deal with human–polar bear conflict. In addition, the human population is increasing across the Arctic. For example, the population in Nunavut, Canada, is projected to grow by 31% from 2014 to 2035 (Heleniak, Reference Heleniak2020). Therefore, waste will increase, as will the potential for human–polar bear conflict associated with anthropogenic food.
Historically, the attraction of polar bears to anthropogenic food has been uncommon because of infrequent overlap of polar bear feeding areas with human settlements. Although polar bears are usually born on land, they spend most of their lives far from people, on sea ice, where they feed on marine mammals that provide an energy-rich diet (Stirling & McEwan, Reference Stirling and McEwan1975; DeMaster & Stirling, Reference DeMaster and Stirling1981). Polar bears are largely driven ashore as a result of the annual cycle of melting sea ice or in search of maternal denning habitats (Knudsen, Reference Knudsen1978; Latour, Reference Latour1981), during which time they use up their fat stores as a source of energy (Derocher et al., Reference Derocher, Nelson, Stirling and Ramsay1990; Ramsay et al., Reference Ramsay, Nelson and Stirling1991). In general, polar bears do not continue to use anthropogenic food once sea ice becomes available again (Lunn & Stirling, Reference Lunn and Stirling1985).
As the global climate warms, the temporal and spatial overlap between polar bears and people is increasing. Reductions in both sea-ice habitat and prey availability are predicted to become more prevalent across broader reaches of the Arctic (Stirling & Derocher, Reference Stirling and Derocher2012). Consequently, polar bears will be drawn to anthropogenic food in more locations across the Arctic and for longer periods, thus threatening their survival and human safety (Rode et al., Reference Rode, Wilson, Regehr, St Martin, Douglas and Olson2015b). This growing conflict will be most prominent in locations where human settlements already overlap with areas where polar bears wait for sea-ice formation. In some regions of their range, polar bears are now spending more time waiting on shore for the sea ice to freeze than before 2000 (Castro de la Guardia et al., Reference Castro de la Guardia, Myers, Derocher, Lunn and Terwisscha van Scheltinga2017). Consequently, polar bears are now spending more time searching for alternative food sources while land-bound. Naturally occurring terrestrial foods, such as beached whale carcasses, bird eggs and fish, are too limited in distribution and abundance to substitute for the usual marine mammal diet of polar bears (Rode et al., Reference Rode, Wilson, Regehr, St Martin, Douglas and Olson2015b). Therefore, polar bears will ultimately be drawn into settlements where nutrient-dense anthropogenic food is often readily available. This is particularly true in areas where once-small settlements and associated dumps are now expanding (Odynova, Reference Odynova2019). The unprecedented polar bear incursion into Novaya Zemlya underscores how quickly a shift in resource use and dependence can arise. Because these situations have high potential for human–polar bear conflict, resulting in both human injury and the removal of polar bears from declining subpopulations, the negative implications of anthropogenic food for polar bear conservation is a growing concern.
We recommend that localities implement the following actions to address this growing problem: (1) better document incidents of food-seeking behaviour (i.e. monitor the number of polar bears seeking food at dumps and other sources), (2) examine the patterns and timing of food-seeking behaviour, (3) document the age and sex composition of food-seeking polar bears, (4) test methods for keeping food from polar bears, (5) assess the effectiveness of deterrence methods used on food-seeking polar bears, (6) assess the efficacy of relocation methods (e.g. helicopter relocation), (7) examine recidivism rates amongst food-seeking polar bears (i.e. food conditioning), and (8) develop a means of predicting when polar bears could seek anthropogenic food (e.g. sea-ice break-up, ice formation, body condition).
Acknowledgements
This paper developed from a meeting in Churchill, Manitoba, in autumn 2019 where the issue of dump use by polar bears arose. We thank Dan Cox for suggesting exploration of this issue and Polar Bears International for arranging this meeting. This study did not receive funding from any agency.
Author contributions
All authors contributed to the various components of this work.
Conflicts of interest
None.
Ethical standards
No animals were handled during this study. Research conducted in this paper complies with all ethical standards in the jurisdictions of the authors and abided by the Oryx guidelines on ethical standards.




