Considerable conservation interest is being paid to Madagascar’s unique biodiversity as the island’s forests shrink, fragment and degrade (Dufils, Reference Dufils, Goodman and Benstead2003). Because of the difficulty of detecting rare or elusive carnivores (Karanth et al., Reference Karanth, Nichols, Kumar and Thompson2004) the majority of ecological surveys conducted in Madagascar exclude or inadequately assess carnivore abundance. Seven of eight species of Malagasy carnivores are categorized on the IUCN Red List (IUCN, 2009; Endangered: giant-striped mongoose Galidictis grandidieri; Vulnerable: fossa Cryptoprocta ferox, narrow-striped mongoose Mungotictis decemlineata, brown-tailed mongoose Salanoia concolor; Near Threatened: Malagasy small-toothed civet Eupleres goudotii, Malagasy civet Fossa fossana, broad-striped mongoose Galidictis fasciata). Despite the declining status of most of these species, only a few short-term assessments of Malagasy carnivores have been completed in the eastern rainforests (Dunham, Reference Dunham1998; Kerridge et al., Reference Kerridge, Ralisoamalala, Goodman, Pasnick, Goodman and Benstead2003).
Camera-trapping is a non-invasive tool used to study carnivores (Karanth et al., Reference Karanth, Nichols, Kumar and Thompson2004). Despite the potential utility of this tool for rapid biological inventories in Madagascar, to our knowledge no camera-trapping studies of carnivores have been conducted there. Our objectives were to evaluate the efficacy of this method for studying Malagasy carnivores and estimate relative abundance and density of carnivores in the eastern rainforest of Ranomafana National Park.
The 43,500 ha, mountainous Ranomafana National Park (Fig. 1) lies at altitudes of 400–1,374 m. Climate is subtropical with a mean annual rainfall of 2,300–4,000 mm. Our survey was during the austral winter, which is characterized by the lowest temperatures of the year (11–17°C) and lowest mean monthly rainfall (90 mm; Karpanty, Reference Karpanty2006).
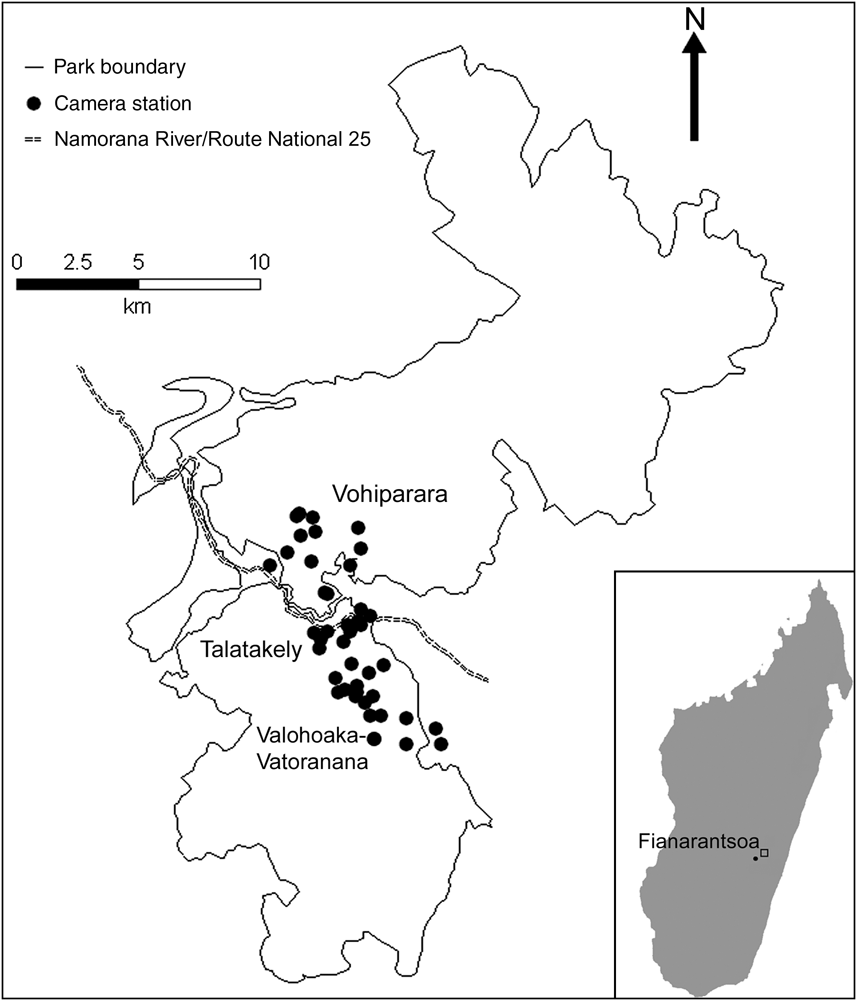
Fig. 1 The locations of 43 camera-stations along three trail systems in Ranomafana National Park in the Fianarantsoa province in south-east Madagascar (see inset for location). The fast-flowing Namorana River and the Route National 25 highway (which run adjacent to each other and are represented by only one line type) separate the Vohiparara trail system from the Talatakely and Valohoaka–Vatoranana trail systems.
We placed 43 camera-trap stations over a total area of 33.5 km2 along three trail systems within the Park (Fig. 1): 23 camera-stations from 6 June to 3 August 2007 in the Vohiparara and Talatakely trail systems, which include secondary and selectively-logged rainforest, and 20 stations from 5 August to 27 August 2007 in the Valohoaka–Vatoarana trail system, which includes a mixture of undisturbed and selectively-logged rainforest. We located stations opportunistically along research trails, with a mean distance of 494 ± SD 294 m between neighbouring stations. Each station consisted of two independently-operating DeerCam DC300 cameras (Non-Typical Inc., Park Falls, USA) mounted on opposite sides of the trail, facing each other. We placed cameras 0.3 m above the ground and set them to be active for 24 h day-1 with a 30-second delay between consecutive photographs. To increase the probability of photo-capture we baited all stations equally with 0.5 kg of chicken meat. We replaced film, batteries and bait, if necessary, every 7 days.
We calculated trap success (capture events/trap nights), which is widely used as a measure of relative abundance in camera-trapping studies for species in which individuals cannot be identified (Kelly, Reference Kelly2008), for each carnivore species. To estimate population size we used closed population capture–recapture analyses for those carnivore species that were identifiable individually. The importance of correcting raw counts by incorporating sampling detection is recognized as essential to accurate estimation of abundance (Nichols, Reference Nichols1992). Thus we evaluated capture histories using seven models, in which different variables affect the detection process: null, individual heterogeneity, time, behaviour (trap-happy vs trap-shy), and mixed combinations (Otis et al., Reference Otis, Burnham, White and Anderson1978).
We calculated density in two ways: (1) traditional capture–recapture analyses using either software MARK (White & Burnham, Reference White and Burnham1999) or CAPTURE (Otis et al., Reference Otis, Burnham, White and Anderson1978) to model the detection process, with camera-trap stations buffered by full mean-maximum-distance-moved among multiple captures in the survey (MMDM) and ½ MMDM (Dillon & Kelly, Reference Dillon and Kelly2008) to estimate effective survey area, and (2) a spatially-explicit maximum-likelihood (SEML) method using software DENSITY (Efford et al., Reference Efford, Dawson and Robbins2004). Survey periods and areas were grouped together to produce an adequate sample for capture–recapture analyses (Di Bitetti et al., Reference Di Bitetti, Paviolo and De Angelo2006).
A total of 755 camera-trap nights provided 1,605 photographs of four endemic carnivore species (C. ferox, F. fossana, ring-tailed mongoose Galidia elegans, G. fasciata), the exotic Indian civet Viverricula indica, and the domestic dog Canis familiaris (Table 1). E. goudotii was the only native carnivore species previously observed within Ranomafana National Park by other researchers (P. Wright, pers. comm.) but was not detected in this study. Of the native carnivores F. fossana and G. elegans had the highest relative abundances (Table 1).
Table 1 Summary statistics of carnivore camera-trapping on the Talatakely, Vohiparara and Valohoaka–Vatoranana trail systems in the rainforests of Ranomafana National Park, Madagascar (Fig. 1), during June–August 2007.
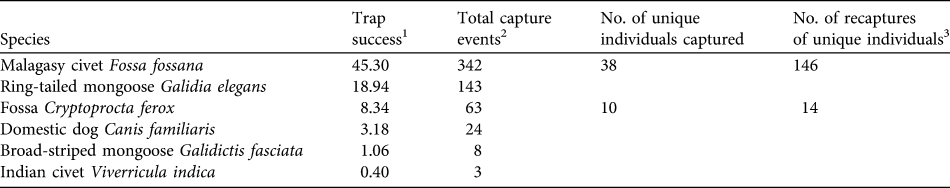
1 A measure of relative abundance for all 43 camera-trap stations calculated as (total capture events / 755 trap nights) * 100. Number of trap nights was defined as the total number of complete 24-hour periods during which at least one of the two cameras at a station was functioning.
2 All photographs taken within a 30-minute period were considered one capture event (Di Bitetti et al., Reference Di Bitetti, Paviolo and De Angelo2006)
3 The total number of sampling occasions on which identified individuals were detected after first capture. To obtain a minimum detection probability of 0.10 for capture–recapture analyses (Otis et al., Reference Otis, Burnham, White and Anderson1978) sampling occasions were calculated as 1-day periods for F. fossana and 3-day periods for C. ferox. We found no evidence of violation of the closed population assumption (C. ferox: χ2 = 7.62, P = 0.37; F. fossana: χ2 = 12.80, P = 0.31; Stanley & Burnham, Reference Stanley and Burnham1999).
Using F. fossana’s pelage markings (spot size, shape and spacing), we individually identified 83% of capture events and constructed individual capture histories for this species. We identified 62% of capture events for C. ferox using slight pelage wear-and-tear patterns. We consider the C. ferox density estimate to be a conservative minimum estimate because some of the 38% of capture events in which we could not identify individuals could be additional animals, which would increase the density estimate for this species.
We found that the capture–recapture history of the individually-identified F. fossana was best explained by incorporation of capture variation among individuals (heterogeneity effect) and an effect of baiting (behaviour effect) into the model (Table 2). Our calculated density of F. fossana was similar using the SEML method and the full MMDM buffered survey (Table 3).
Table 2 Candidate models in Akaike Information Criterion (AIC) model selection procedure used to best-fit capture–recapture histories of F. fossana from 43 camera-trap stations on the Talatakely, Vohiparara and Valohoaka–Vatoranana trail systems (Fig. 1) during June–August 2007.
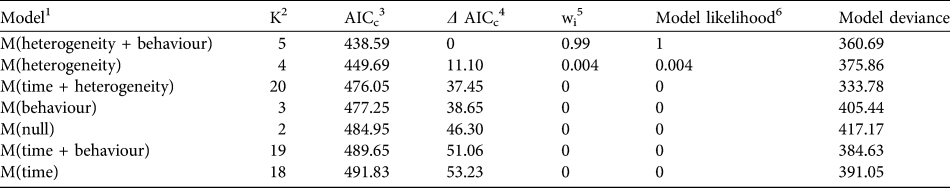
1 Capture histories evaluated by modelling the detection process, after Otis et al. (Reference Otis, Burnham, White and Anderson1978)
2 Number of parameters per model
3 AIC with small sample bias adjustment (Burnham & Anderson, Reference Burnham and Anderson1998)
4 Difference between a model’s AICc and the best-fitting model
5 Percentage of model weight attributed to each model
6 Strength of evidence of each model relative to other candidate models
Table 3 Abundance and density estimates for F. fossana and C. ferox using two types of mean-maximum-distance-moved buffer values (½ MMDM and MMDM) and their associated effective survey areas, and a maximum-likelihood spatially-explicit model (SEML; Efford et al., Reference Efford, Dawson and Robbins2004) using data from 43 camera-trap stations on the Talatakely, Vohiparara and Valohoaka–Vatoranana trail systems (Fig. 1) during June–August 2007.
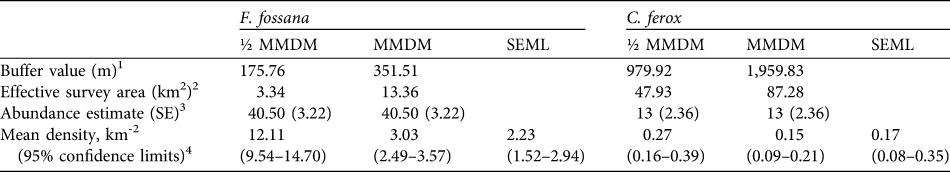
1 Calculated assuming a circular buffer around each camera-station (Dillon & Kelly, Reference Dillon and Kelly2008)
2 The buffers around each camera-station dissolved together give the survey area of the entire study grid
3 F. fossana model-averaged estimate using MARK (White & Burnham, Reference White and Burnham1999), and C. ferox estimate based on the Jackknife-M(heterogeneity) of CAPTURE (Otis et al., Reference Otis, Burnham, White and Anderson1978)
4 Calculated by dividing the abundance estimate by the effective survey area, with variance calculated following Dillon & Kelly (Reference Dillon and Kelly2008). SEML estimates derived from DENSITY (Efford et al., Reference Efford, Dawson and Robbins2004; for F. fossana based on hazard rate Mth, and for C. ferox based on half-normal M(null)).
The low number of identified individuals of C. ferox (Table 1) prohibited an effective use of MARK and therefore we used CAPTURE, which selected the null model as the most parsimonious (criterion = 1.0) and individual heterogeneity secondly (criterion = 0.88). We used the latter to calculate abundance as it is more biologically realistic and robust to violations (Boulanger & Krebs, Reference Boulanger and Krebs1996). As with F. fossana, we found that C. ferox density estimates were similar using an effective survey area buffered by full MMDM and the SEML method (Table 3). Our minimum density estimates of C. ferox (Table 3) are lower than estimates of the species in western deciduous dry forest (0.26 km-2; Hawkins & Racey, Reference Hawkins and Racey2005).
Each of the four native carnivores (F. fossana, G. elegans, C. ferox, G. fasciata) photographed were found in all three trail systems. This suggests that the variation in disturbance history across our study area, from selectively-logged to undisturbed forests, did not alter carnivore species richness. Two individuals each of C. ferox and F. fossana were detected moving from Talatakely to the Valohoaka–Vatoarana trail systems but such movement was not detected between Vohiparara and Talatakely. This suggests that the Namorana River and/or national highway (RN25) may act as barriers to carnivore movement between the northern and southern parts of the Park (Fig. 1).
Two exotic carnivore species, V. indica and C. familiaris, were detected. V. indica was only observed at two camera-trap stations, within 500 m of the highway. C. familiaris was detected at 16 of the 43 stations in Talatakely and Vohiparara. Subsequent observations have also confirmed the presence of dogs on the Valohoaka-Vatoarana trail system (B. Gerber, pers. obs.).
This study provides the first density estimates for carnivores in the eastern rainforests of Madagascar and suggests that the density of C. ferox in Ranomafana National Park is lower than that of conspecifics in the western dry forests. We found that camera-trapping is an efficient, non-invasive tool to quantify relative abundance of Malagasy carnivores and density of the two largest species, F. fossana and C. ferox. We have now begun a larger-scale study to address the question of how carnivore density varies with forest disturbance in the larger rainforest complex in south-east Madagascar.
Acknowledgements
Funding was provided by Virginia Tech. We thank the Government of Madagascar, the Association Nationale pour la Gestion des Aires Protégées, the Direction des Eaux et Forêts, and CAFF/CORE for permission to conduct this research. We were greatly assisted by ICTE/MICET, Centre ValBio, and Amelaid Houmadi. Comments from two anonymous reviewers greatly improved this article.
Biographical sketches
Brian Gerber has worked since 1999 in field studies of primates and predators. Sarah Karpanty has worked in Madagascar since 1997 on predator-prey ecology in fragmented landscapes. Charles Crawford, now pursuing graduate studies in predator ecology, and Johnny Randrianantenaina, a research assistant at Centre ValBio, conducted the majority of the fieldwork reported here. Mary Kotschwar is currently studying the behavioural responses of lemurs to predation risk.






