Introduction
Most of our vitamin D is produced via exposure of the skin to UV radiation through sunlight exposure, with relatively small amounts also provided by dietary sources. Due to our reliance on the sun for most of our vitamin D, it has been known as the ‘sunshine vitamin’ in the past, which is an especially relevant name for vitamin D in a sunny country such as Australia, where it has been estimated that the population receives 90–95 % of vitamin D from the sun's UV radiation(Reference Nowson, Diamond and Pasco1). This creates a dilemma whereby our main source of this essential vitamin is via a known carcinogen that has been shown to increase the risk of skin cancers and is associated with other detrimental effects such as eye damage(Reference Godar2). Foods contribute only relatively small amounts of vitamin D; however, useful amounts of vitamin D are found in foods such as fortified margarine (these are mandatorily fortified in Australia), fortified milks, eggs, and fish species such as North Sea salmon, herring and mackerel(Reference Shrapnel and Truswell3).
Due to Australia being considered a sunny country, vitamin D deficiency (defined as serum 25-hydroxyvitamin D (25(OH)D) < 50 nmol/l) and insufficiency (50–75 nmol/l), until recently, was only thought to be a major health problem in certain population groups such as the institutionalised elderly. Recent studies, however, have shown that vitamin D insufficiency is more widespread than first thought(Reference McGrath, Kimlin and Saha4, Reference Pasco, Henry and Nicholson5). While severe vitamin D deficiency causes rickets in children and osteomalacia in adults, vitamin D deficiency is also associated with an increased risk of osteoporosis and falls, as well as emerging evidence that vitamin D may play a role in reducing the risk of certain cancers, autoimmune diseases and hypertension(Reference McGrath, Kimlin and Saha4).
In the first part of this review paper we present an overview of the two sources of vitamin D, i.e. UV radiation and diet, and investigate the controversies surrounding both of these sources. The second part of this paper provides a review of the evidence in regard to vitamin D's role in human health, with a particular focus on its role in bone health, muscle strength and cancer. While the present paper has an Australian emphasis, the implications of vitamin D deficiency and ongoing dilemma of our main source, UVB radiation, also being a human carcinogen, ensure an international relevance.
Vitamin D nomenclature
As vitamin D exists in a number of forms and these terms are used throughout the present review, an introduction to these major forms of vitamin D is necessary. Table 1 provides an overview of the four main forms of vitamin D discussed in the present review. Ergocalciferol (vitamin D2) is produced by UV irradiation of ergosterol in plants and cholecalciferol (vitamin D3) is produced by UV irradiation of 7-dehydrocholesterol in the skin of vertebrates(6). Both 25(OH)D and 1,25-dihydroxyvitamin D (1,25(OH)2D) are produced by subsequent hydroxylation of cholecalciferol in the human body and are the main circulatory and physiologically active forms in the human body, respectively. It is worth noting that activated forms of vitamin D such as 1,25(OH)2D are rarely used in clinical settings due to the higher risk of hypercalcaemia(Reference Johnson and Kimlin7).
UV radiation
Solar radiation consists of a continuous spectrum of radio, microwaves, X-rays, visible, IR and UV radiation(Reference Maddodi and Setaluri8). Of these, the UV spectrum receives the most attention due to both its positive and negative effects on human health(Reference Maddodi and Setaluri8). A range of UV wavebands exists, although only the UVA (320–400 nm) and UVB (290–320 nm) wavebands reach the earth's surface, with the extreme, far and UVC wavebands being filtered out by oxygen and ozone before reaching the earth's surface(Reference Godar2). The main beneficial effect of UV exposure is the photoproduction of vitamin D synthesis, while negative effects of UV exposure include melanoma and non-melanoma skin cancers and eye damage.
UV radiation and health
UV radiation and skin cancer
UV radiation exposure is associated with a number of negative health effects, the most important and certainly most researched being the various forms of UV-induced skin cancers. The typical skin cancers associated with exposure to UV radiation are basal cell carcinomas, squamous cell carcinomas and malignant melanomas(Reference van Doorn, Gruis and Willemze9). Of these, basal cell carcinomas are the most common, making up approximately 70–85 % of all skin cancers, while squamous cell carcinomas account for about 15–20 % and malignant melanomas about 5 % of skin cancers in Australia(10). Malignant melanoma is the most dangerous form of these skin cancers and is responsible for the majority of deaths from skin cancers(Reference MacKie11). UV exposure appears to be the main risk factor for both basal and squamous cell carcinomas, while other important risk factors as well as UV, such as genetics and number of naevi, exist for malignant melanoma(Reference Godar2).
Skin cancer is of particular concern in Australia, due to high annual sunshine and the major role outdoor activity plays in many people's lifestyles generally. Thus, it comes as no surprise that Australia has the highest rate of skin cancer in the world, with one in two Australians being diagnosed with skin cancer in their lifetime and over 1600 deaths being attributable to skin cancer each year in Australia(12). Sun protection messages and promotional campaigns such as Slip Slop Slap and Sunsmart have attempted to reduce this high rate of skin cancer, by promoting sun-safe messages such as avoidance of the sun between 10.00 and 15.00 hours and the use of a sun protection factor 30+sunscreen(13). Recent evidence seems to show that they may be having the desired effect, with reported reductions in rates of non-melanoma skin cancer reported in individuals aged under 50 years, at least partly attributable to these campaigns(Reference Lucas, Repacholi and McMichael14).
Mechanisms of UV carcinogenesis and mutagenesis
Both UVA and particularly UVB radiation exposure appears to contribute to the risk of developing skin cancers through different mechanisms(Reference Lucas, Repacholi and McMichael14). UVB radiation, whose photons are absorbed by DNA, has been shown to exert carcinogenic effects via production of photoproducts from DNA bases such as cyclobutane pyrimidine dimers and pyrimidine–pyrimidone (6-4) photoproducts, which exert carcinogenic effects on the epidermis(Reference Maddodi and Setaluri8). Additional carcinogenic effects of UVB include suppression of immune function and up-regulation of gene expression which may enhance tumour development(Reference Ichtihashi, Ueda and Budiyanto15). UVA radiation appears to induce carcinogenic effects indirectly via reactive oxygen species, which cause DNA breaks and oxidation of nucleic acid bases(Reference Marrot and Meuenier16). An area of particular interest is the recent research into the UV-induced mutation (at bipyrimidine sites) of the p53 tumour-suppressor gene, which is responsible for regulating DNA repair and apoptosis(Reference Weidong, Ananthaswarmy and Muller17). Mutations in this gene inhibit its ability to carry out DNA repair or apoptosis of damaged cells, increasing the risk of tumour development(Reference Marrot and Meuenier16).
Epidemiological evidence for the role of UV in skin cancers
Epidemiological evidence suggests a strong relationship between UV radiation exposure and non-melanoma skin cancers(Reference Rigel18). The epidemiological evidence for UV radiation and malignant melanoma is rather more complex, as other factors such as genetics also play a role in melanoma development(Reference Godar2). There is strong evidence linking average annual UVB exposure to melanoma risk; however, melanoma risk association using latitude gradient studies has provided much more inconsistent evidence(Reference Rigel18). While it is acknowledged that UV exposure plays the greatest role in determining melanoma risk in European populations, gaps in the research do exist in regard to the role of UV radiation in the aetiology of malignant melanoma(Reference Maddodi and Setaluri8, 19). Animal and in vitro models have contributed much to our understanding of the carcinogenic effects of UV radiation. However, with UV radiation having an impact on many biological processes linked to carcinogenesis and mutagenesis in man, the full picture is far from complete and further major discoveries to advance our knowledge in this field are certain.
Other health effects of UV radiation
Although the main focus of this section has been the relationship between UV radiation and skin cancer, UV radiation also has an impact on other areas of health. UV radiation exposure is thought to be a major cause of eye damage, although the epidemiological evidence is only sufficient to confirm a causal association with photokeratitis (snow blindness), with more limited evidence for pterygium, cataracts, climatic droplet keratopathy and anterior lens capsule changes(Reference Johnson20). Other negative effects of UV radiation include photoageing and sunburn(Reference Godar2).
UV radiation and vitamin D
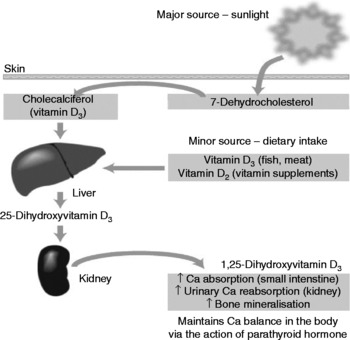
While there are a number of deleterious effects of UV radiation exposure on the human body, of which skin cancers are the most severe, there are also benefits in the form of vitamin D synthesis. UVB radiation (in the 280–320 nm spectrum) is the main source of vitamin D for man(Reference Kimlin, Olds and Moore21). The UV radiation catalyses a reaction in the human epidermis whereby 7-dehydrocholesterol is converted to cholecalciferol (vitamin D3), in a two-step process, which then undergoes two hydroxylations in the liver and kidney to produce the active form of the vitamin: 1,25-dihydroxycholecalciferol(Reference Nowson and Margerison22). In this way an estimated 90–95 % of our vitamin D (at least in Australia) is formed, with dietary sources making up the balance(Reference Lips23). Fig. 1 provides a simplified version of vitamin D production in the human body.
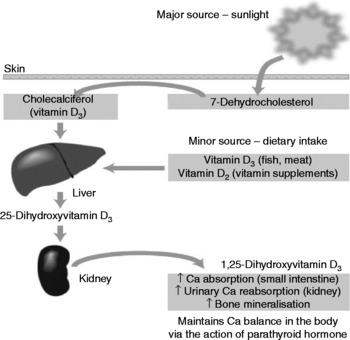
Fig. 1 Vitamin D sources and activation (from Nowson & Margerison(Reference Nowson and Margerison22)).
Vitamin D production in human skin is determined by a number of factors such as time of day, latitude, season, skin colour and age. Individuals with more darkly pigmented skin are at higher risk of vitamin D deficiency due to melanin competing with 7-dehydrocholesterol for UV absorption(Reference Armas, Dowell and Akhter24). Vitamin D production is also less efficient in the elderly due to age-associated decreases in skin 7-dehydrocholesterol(Reference Wolpowitz and Gilchrist25). Latitude, season and time of day also influence vitamin D synthesis via the solar zenith angle, which affects the amount and distribution of solar radiation reaching the earth's surface, i.e. small solar zenith angles are associated with summer, noon and latitudes closer to the equator, thus increasing skin exposure to UVB radiation and vitamin D synthesis(Reference Webb and Engelsen26). However, recent research has shown that erythemal UV latitude gradients may not be as important as once thought for vitamin D synthesis and that other factors, such as total ozone, cloud cover, aerosols, surface reflectivity and altitude, that also affect vitamin D production in the skin may play a greater role(Reference Engelsen, Brustad and Aksnes27).
Required levels of sun exposure for adequate vitamin D synthesis
There is a major concern that with public health messages focusing on reducing sun exposure, individuals are not producing enough vitamin D. Indeed, even in sunny Queensland, Australia, which is the known as the ‘skin cancer capital of the world’, a recent study showed 42·5 % of participants were vitamin D deficient in winter(Reference Kimlin, Harrison and Nowak28). This creates a dilemma, whereby individuals avoiding UV exposure to significantly reduce their risk of skin cancers and other harmful UV effects may actually also be increasing their risk of vitamin D insufficiency and even deficiency. Therefore it is of great interest for researchers to find out how much UV exposure is needed to produce adequate vitamin D and whether there is a safe level of UV exposure. Samanek et al. (Reference Samanek, Croager and Gies29) attempted to shed light on this issue with an ecological study design using UV index data collected from major Australian population centres in a 1-year study. This study used a model based on Fitzpatrick type-II skin types (white skin that always burns easily), with a 15 % skin exposure and between one-sixth to one-third of a minimal erythemal dose (which is the time taken for UV radiation to cause a slight reddening of the skin); this minimal erythemal dose figure was the calculated UV dose needed to produce adequate vitamin D(Reference Samanek, Croager and Gies29). For example, in January, in Brisbane, Australia, between 2 and 4 min sun exposure per d was needed during summer months, at noon, to produce enough vitamin D, based on daily vitamin D requirements(Reference Samanek, Croager and Gies29).
Obviously, seasonal changes and time of exposure changed the amount of sun exposure needed and increased exposure times were needed in population centres with lower latitudes. There were numerous weaknesses in the study; for example, it did not take into account factors such as ageing that affect vitamin D synthesis, was conducted over a relatively short length of time and only provided recommendations for one skin type. Despite these weaknesses, it provides important information on doses of sun exposure needed for adequate vitamin D synthesis while avoiding dangerous overexposure to the sun. Other studies(Reference Webb and Engelsen26, Reference Terenetskaya30) have also provided data on estimated UV exposure times required for vitamin D synthesis using in vitro systems such as spectrophotometric analysis of the previtamin D content in ethanol solutions of 7-dehydrocholesterol during UV exposure. A particular strength of the study by Webb & Engelsen(Reference Webb and Engelsen26) was that it predicted sun exposure times needed for adequate vitamin D synthesis for different skin types.
It may be some time before we are able to accurately predict ‘safe’ levels of sun exposure that result in adequate vitamin D synthesis, but do not result in increased risk of skin cancers if, indeed, such an ideal exists. While research, does, so far, show us how complex UV exposure and vitamin D research really is, the Cancer Council of Australia has released broad recommendations on how to achieve adequate vitamin D production while remaining ‘sunsmart’(31). For instance, recommendations made by the Cancer Council of New South Wales state that exposing face, hands and arms for 10 min in summer, 15–20 min in spring and autumn and 30 min in winter outside the peak UV times (10.00–15.00 hours) should be adequate to produce enough vitamin D(32).
Vitamin D and diet
Vitamin D food sources
Dietary intake contributes only small amounts of vitamin D overall, with vitamin D found in reasonable amounts in North Sea oily fish such as salmon, herring, sardines and tuna (4–25 μg/100 g), egg (1·8 μg/100 g), butter (1·4 μg/100 g) and red meat and liver (0·7–1·1 μg/100 g)(Reference Shrapnel and Truswell3). The vitamin D within the same food products may also vary, with recent research showing that the vitamin D content of fish may vary enormously depending on whether fish is farmed or wild. For instance, Chen et al. (Reference Chen, Chimeh and Lu33) analysed the vitamin D content of both wild and farmed salmon and discovered that wild salmon contained four times the vitamin D content of farmed salmon. Despite the relatively low amount of dietary vitamin D found naturally in foods, there is a growing interest in the role of vitamin D-fortified foods and supplements in improving vitamin D status.
Vitamin D dietary intake in Australia
In regard to the actual amount of vitamin D we receive from food, it is apparent that vitamin D intake in Australia is low, with study data showing that Australians on average only consume 2–3 μg vitamin D per d from dietary sources(Reference Nowson and Margarison34). This shows that, on average, Australians are well below meeting the 5, 10 and 15 μg dietary vitamin D per d recommended for < 50, 50–70 and 70+years age groups, respectively(35). Another interesting finding from this study was that, on average, 50 % of the Australian population's vitamin D intake is sourced from fortified margarine products(Reference Nowson and Margarison34). This study formed part of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) National Diet Survey and used a semi-quantified FFQ sent out in two batches of 5000 postal surveys to randomly selected households from the electoral roll in 1998 and 1999(Reference Nowson and Margarison34). Limitations included the lack of any data collected on vitamin D supplements and the relatively low participation rate of 43 %. However, this study is, to date, the only comprehensive nationwide survey that has collected information on dietary vitamin D intake in Australia, so the data collected are of great significance.
Only a few other studies have attempted to collect information on dietary vitamin D intake in Australians. Jones et al. (Reference Jones, Blizzard and Riley36) and A Sherwin and C Nowson (unpublished results) collected data on the vitamin D intake of 201 children with a FFQ and 215 females in residential care or nursing homes using a plate-waste survey, respectively(Reference Nowson and Margerison22). These two studies reported dietary vitamin D intakes that were approximately half of those recorded in the Commonwealth Scientific and Industrial Research Organisation (CSIRO) National Diet Survey (1 μg/d and 0·9 μg/d (median), respectively), which is to be expected due to the lower food intakes associated with children and residential or nursing home residents. Another contributor to the lower reported intake in the study by Jones et al. (Reference Jones, Blizzard and Riley36) would almost certainly be the lack of data collected on margarine, which, as mentioned previously, is a major contributor to dietary vitamin D intake in Australia(Reference Nowson and Margarison34). Pasco et al. (Reference Pasco, Henry and Nicholson5) also collected data on the vitamin D intake of women ranging in age from 20 to 92 years in a much larger study (n 861, randomly selected), as part of the Geelong Osteoporosis study and found median intakes of 1·2 μg/d, using an FFQ. This study also reported that 7·9 % of participants used vitamin D supplements, which resulted in an increase to the median vitamin D intake of 0·1 μg/d (for a total of 1·3 μg/d)(Reference Pasco, Henry and Nicholson5). The findings from this study showed a significant association between dietary vitamin D intake and vitamin D status in winter, but no significant association between these two variables in summer. It also highlighted the limited use of vitamin D supplements in this group, although further studies into vitamin D supplement use are needed to confirm whether this is common to other parts of Australia or has changed over the past 7 years(Reference Pasco, Henry and Nicholson5).
International vitamin D dietary intake
International studies into dietary vitamin D status have shown quite varied results, reflecting national trends in food consumption and the use of food fortification(Reference Park, Murphy and Wilkens37–Reference Morabia, Bernstein and Antonini43). However, comparison between different countries is very difficult, due to the wide variety of different FFQ food recall times used, which can range from 3 d to 1 year. Also, some studies, such as the large Multi Ethnic Cohort study (n 191 011) measured supplement intake of vitamin D in participants, while others such as the UK European Prospective Investigation into Cancer and Nutrition (n 65 469) did not(Reference Park, Murphy and Wilkens37, Reference Roddam, Neale and Appelby39). Additionally, many studies also reported means (which may reflect extreme values) rather than medians. Table 2 shows the reported vitamin D intakes from various international studies.
Table 2 International dietary vitamin D intakes*

* Adapted from Nowson & Margerison(Reference Nowson and Margerison22).
† All values are means unless otherwise stated.
The data presented in Table 2(Reference Park, Murphy and Wilkens37–Reference Morabia, Bernstein and Antonini43) show intakes averaging 1–3 μg/d in studies from the UK, Tunisia, Turkey and Switzerland, which is similar to the Australian intakes reported above(Reference Roddam, Neale and Appelby39–Reference Gannage-Yared, Chemali and Yaacoub41, Reference Morabia, Bernstein and Antonini43). However, dietary intake of vitamin D appears higher in the two studies investigating reported intake of vitamin D in the USA and the study from Finland(Reference Park, Murphy and Wilkens37, Reference Wang, Manson and Buring38, Reference Lamberg-Allardt, Outila and Karkkawen42). This may be partially explained by the fact that these three studies reported supplemental intake of vitamin D while the other studies presented in Table 2 did not. Also, the more widespread food fortification practices used in both the USA and Finland are primary predictors of dietary vitamin D intake in these countries, with intakes 60 % higher in these countries than in New Zealand, where vitamin D fortification of the food supply is at a similar level to Australia(Reference MacKie11).
Vitamin D food fortification
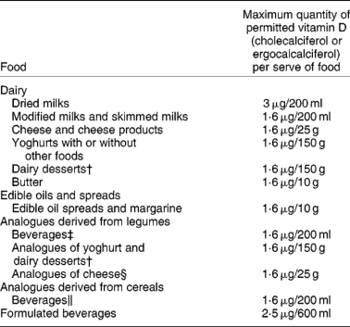
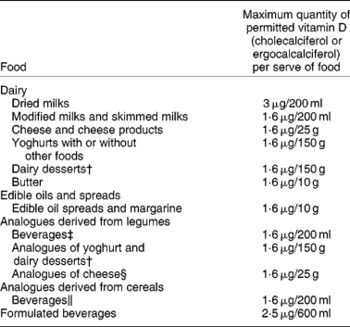
Fortification practices in different countries are varied. For example, in Australia we mandatorily fortify margarine, and food manufacturers can voluntarily fortify some foods such as modified milks and skimmed milks with ergocalciferol, which in practice is not used, or cholecalciferol, but few of these vitamin D-fortified products are available(Reference Nowson and Margerison22, 44). Foods allowed for fortification and the quantity of cholecalciferol or ergocalciferol allowed, as outlined by the Australian and New Zealand Food Standards Code, are presented in Table 3.
Table 3 Maximum quantity of ergocalciferol or cholecalciferol fortification allowable in permitted Australian and New Zealand foods*
* The source of data was Food Standards Australia New Zealand(44).
† Containing no less than 3·1 % (w/w) of protein derived from milk protein.
‡ Containing no less than 3·1 % (w/w) of protein derived from legume protein.
§ Containing no less than 15 % (w/w) protein derived from legumes.
∥ Containing no less than 0·3 % (w/w) protein derived from cereals.
The UK mandatorily fortifies margarine with a fat content of 80 % or above, while in the USA only milks labelled as ‘fortified’ have to actually be fortified with vitamin D; however, more products are able to be fortified in both the USA and the UK, such as breakfast cereals(Reference Nowson and Margerison22). Indeed, breakfast cereals in the UK have been shown to provide 13 % of the mean intake of vitamin D for adults, thus contributing a major source of vitamin D in the UK(Reference Henderson, Irving and Gregory45). Both Canada and Finland have introduced mandatory fortification of milk and margarine and in Finland at least, this seems to have had a positive impact on vitamin D status, at least during winter(Reference Laaksi, Ruohola and Ylikomi46). For instance, a study on a group of young Finnish males showed that 78 % had vitamin D levels below 40 nmol/l, but after 1 year of fortification being introduced, only 35 % of them had vitamin D levels under 40 nmol/l; furthermore, there was a mean 50 % increase in vitamin D levels(Reference Laaksi, Ruohola and Ylikomi46). This study shows the significant effect that food fortification can have on improving vitamin D status in a country where sun exposure is limited, especially during winter. More widespread fortification may also have a more important role in maintaining adequate vitamin D status in the future for sunny countries such as Australia.
Vitamin D and determinants of health
Vitamin D receptors (VDR) are present on many tissues and organs in the body and as such vitamin D has been identified as playing a role, or a potential role, in many human diseases and conditions. Vitamin D's role in bone health is well known, with osteomalacia and rickets being the traditional conditions associated with serious vitamin D deficiency. Vitamin D's role in reducing the risk of osteoporosis is also seen as a major function of the vitamin. Emerging research has also highlighted possible roles for vitamin D in reducing the risk of certain autoimmune diseases (for example, type 1 diabetes), some cancers (such as colon cancer), CVD and the maintenance of muscle strength in older adults(Reference Holick47).
With vitamin D's wide range of target tissues and actions, it is not surprising that its involvement in the disease processes of multiple health conditions has been widely researched. The following sections review the major research conducted in regard to vitamin D's role in health and disease.
Optimal vitamin D levels for health
What constitutes a ‘normal’ or ‘optimal’ serum 25(OH)D level for health has been an area of some debate. Although several measures of deficiency and insufficiency exist, commonly used criteria for defining levels of vitamin D deficiency, based on the measurement of serum 25(OH)D, are provided below (Lips(Reference Lips48), ANZBMS Position statement(49) and Holick(Reference Holick50)):
(a) < 12·5 nmol/l: severe;
(b) 12·5–25 nmol/l: moderate deficiency;
(c) 25–50 nmol/l: mild deficiency;
(d) >50–75 nmol/l: insufficiency;
(e) >75 nmol/l: sufficiency.
It is important to note here that serum 25(OH)D status is generally used to define vitamin D status rather than serum 1,25(OH)2D. This may seem counterintuitive; however, serum 1,25(OH)2D is thought to be a poor indicator of vitamin D status, as it is able to be produced locally by many tissues and is not depressed when individuals display mild to moderate serum 25(OH)D deficiency with raised parathyroid hormone (PTH)(Reference Johnson and Kimlin7).
These commonly used criteria are based on the physiological effects that low serum 25(OH)D status induces on bone, Ca absorption and serum PTH. Levels of 50–75 nmol/l, now cited by many authors as vitamin D insufficiency, are associated with decreased Ca absorption and a small increases in PTH release (note that PTH only stabilises at approximately 100 nmol/l)(Reference Holick50). Mild deficiency, also confusingly termed insufficiency by some authors in the past, is associated with higher bone turnover and increases in PTH release, moderate deficiency is associated with increased bone turnover and moderate increases in serum PTH levels, while severe deficiency is associated with high bone turnover leading to osteomalacia and large increases in serum PTH levels(Reference Lips48). There is, however, still some confusion on cut-off levels for determining serum 25(OH)D based on the lack of standardised physiological cut-off points to define deficiency and insufficiency and also the use of different cut-off points by authors for statistical analysis of deficiency and insufficiency in varied population groups.
Definitions to define serum 25(OH)D deficiency and insufficiency have changed over time(Reference Johnson and Kimlin7). Originally the serum 25(OH)D levels of asymptomatic subjects were plotted using Gaussian (normal) distributions and these data were used to define ‘normal’ vitamin D status, but these data are approaching 40 years old and new biomarkers to predict at what level 25(OH)D is sufficient, such as PTH and Ca absorption, are now available(Reference Hollis51). A recent paper by six experts in vitamin D research has attempted to reduce this confusion by determining, in the expert opinion of the authors, the optimal minimal serum 25(OH)D level for fracture prevention(Reference Dawson-Hughes, Heaney and Holick52). The criteria for determining this level included the level of 25(OH)D required for suppression of PTH, optimal bone mineral density (BMD), reduced bone loss, fracture and fall risk. After an extensive review of the literature, five out of the six authors agreed that 70–80 nmol/l was a minimal optimal 25(OH)D level for bone health, with one author estimating 50 nmol/l due to the lack of evidence at this time for the broad population supplementation needed to reach the higher levels(Reference Dawson-Hughes, Heaney and Holick52). This is a valid point, as food intake alone is extremely unlikely to raise concentrations of 25(OH)D to the optimal levels mentioned above in the general population, without widespread supplementation or sun exposure(Reference Dawson-Hughes, Heaney and Holick52).
Some trials with vitamin D supplementation have shown that higher doses of vitamin D, 20 μg (800 IU) daily or 2500 μg (100 000 IU) 4-monthly, are needed to raise serum 25(OH)D levels to a mean of approximately 75 nmol/l and it is these higher levels of supplementation that have been shown to provide the most significant benefits in fall and fracture risk(Reference Dawson-Hughes, Heaney and Holick52). Together these findings seem to support a minimal optimal threshold of 70–80 nmol/l for serum 25(OH)D levels, for the reduction of fracture and fall risk. Thus the use of>75 nmol/l as a definition for sufficient serum 25(OH)D status is used in the present review paper. A weakness in these guidelines, however, is that they refer to optimal serum 25(OH)D levels for bone health and may not apply to other health conditions linked to vitamin D such as cancer. Serum 25(OH)D levels of>75 nmol/l have, however, shown that they may significantly reduce the risk of some types of cancers and there is agreement among many experts that levels of serum 25(OH)D>75 nmol/l may be beneficial for cancer risk reduction(Reference Ingraham, Bragdon and Nohe53). Recommendations of optimal levels of serum 25(OH)D for prevention of other disease unrelated to bone health such as cancer may at this stage be premature, however, due to the relative lack of consistent epidemiological data for other health endpoints associated with serum 25(OH)D status.
Vitamin D and osteoporosis
Osteoporosis is a serious condition associated with a gradual loss of bone density, resulting in weak, brittle bones. In Australia, it is estimated that one in two women and one in three men will have an osteoporotic fracture and every 8 min someone is admitted to a hospital with an osteoporotic fracture(54). Osteoporosis is a disease associated with advancing age due to increasing rates of bone losses, which results in a lower bone density(55). Females are also at a significantly greater risk due to decreases in oestrogen during menopause. Oestrogen stimulates bone remodelling; thus a reduction in oestrogen levels results in increased bone losses through increased bone resorption(55). Other important risk factors for osteoporosis include: fragility or low impact fracture in a first-degree relative, low body weight, smoker, use of corticosteroid medication>3 months, early menopause, low Ca intake, low serum 25(OH)D and decreased physical activity (especially weight bearing)(56). The main sites of fracture involve the wrist, vertebrae and hip and these not only result in significant decreases in mobility and in quality of life for patients, but major fractures are also associated with increased mortality following the fracture(Reference Boonen, Vanderschueren and Haentjens57).
Vitamin D's role in bone health
Severe serum 25(OH)D deficiency is associated with the vitamin D deficiency disease of osteomalacia, which results in defective mineralisation of the osteoid on the cortical and trabecular surfaces of bone(Reference Prentice58). Osteomalacia results in bone and muscle pains and an increased risk of fractures, and although it is a condition thought to be rare in Australia, limited data exist on its prevalence worldwide(49).
The active form of vitamin D, 1,25(OH)2D, is also thought to play an important role in the pathogenesis of osteoporosis through its role in Ca and bone metabolism, via numerous mechanisms. Promotion of active Ca absorption through the intestine via the nuclear VDR by 1,25(OH)2D is one of the most widely understood mechanisms(Reference Lanham-New59). This is especially important when Ca intake is low, but less so when Ca intake is high, which results in a higher passive transport of Ca into the circulation and decreased active, 1,25(OH)2D-mediated, transcellular transport(Reference Bronner60). However, Ca absorption is not the only role that 1,25(OH)2D plays in bone health as it also stimulates bone maturation, matrix formation, renal reabsorption of Ca, bone remodelling and osteoclast cell activity(Reference Lanham-New59). PTH is essential in regulating Ca and vitamin D metabolism through it roles in 1,25(OH)2D activation, maintaining Ca homeostasis and bone resorption(Reference Boonen, Vanderschueren and Haentjens57).
To illustrate the relationship between PTH and serum 25(OH)D, Fig. 2 shows the correlation between reduced serum 25(OH)D levels and higher levels of PTH. It is apparent from this scatterplot that there is a gradual increase in plasma PTH level at serum 25(OH)D levels below 100 nmol/l, while a much steeper increase in plasma PTH can be observed when serum 25(OH)D falls below 40–50 nmol/l(Reference Mosekilde61). This is clinically important, as the resulting increase in PTH increases hydroxylation of 25(OH)D to the active 1,25(OH)2D form(Reference Chapuy, Schott and Garnero62). These higher 1,25(OH)2D levels in turn assist in maintaining normocalcaemia, via increased Ca absorption(Reference Mosekilde61). Bone resorption, which results in increased Ca ion release into the bloodstream, is also stimulated by the increased PTH, causing bone losses and reduced bone density(Reference Boonen, Vanderschueren and Haentjens57).

Fig. 2 Correlation between serum 25-hydroxyvitamin D (25(OH)D) and parathyroid hormone (PTH) in Danish perimenopausal women (from Mosekilde(Reference Mosekilde61)). (- - -), Upper normal range for plasma PTH; ↓ , threshold value for 25(OH)D; (□), plots for individual women.
Many epidemiological studies have been conducted investigating the relationship between vitamin D and osteoporosis and a significant number of these have been high-quality randomised controlled trials (RCT). Studies exploring the relationship between vitamin D and osteoporosis generally either use fractures or indicators of bone density and turnover such as BMD.
Meta-analyses of vitamin D's role in osteoporosis
A meta-analysis conducted by Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Willett and Wong63) found significant relationships between vitamin D supplementation with vitamin D3 and fracture or bone density measures. Birshoff-Ferrari et al. (Reference Bischoff-Ferrari, Willett and Wong63) pooled studies into those using higher vitamin D3 doses of 700–800 IU/d (17·5–20 μg/d) and those using low-dose supplements of 400 IU/d (10 μg/d) and found the higher-dose studies were associated with decreasing hip fracture by 26 % (three RCT with 5572 subjects; pooled relative risk (RR) 0·76; 95 % CI 0·61, 0·88) and non-vertebral fractures by 23 % (five RCT with 6098 subjects, pooled RR 0·77; 95 % CI 0·68, 0·87). No significant results were reported for RCT that used vitamin D3 at 400 IU/d (10 μg/d). Tang et al. (Reference Tang, Eslick and Nowson64) conducted an even larger analysis encompassing seventeen randomised trials and a total of 63 897 participants over 50 years of age with both fracture and BMD as endpoints. This analysis showed that treatment with Ca supplements (with or without vitamin D) was associated with a reduction in fracture risk of 12 % (RR 0·88; 95 % CI 0·83, 0·95; P = 0·0004)(Reference Tang, Eslick and Nowson64). While vitamin D (forms of vitamin D used not reported) did not appear to have a significant role in reducing fracture risk, subgroup analysis revealed that the addition of vitamin D to Ca supplementation did result in a significant treatment effect in those institutionalised compared with the community (RR 0·76 v. 0·94; P = 0·003). Tang et al. (Reference Tang, Eslick and Nowson64) additionally reported that the treatment effect was improved if Ca doses were 1200 mg or higher and vitamin D doses were 20 μg (800 IU) and higher. The results from both of these analyses are extremely informative due to the inclusion of only intervention trials; however, only Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Willett and Wong63) reported a strong dose–response effect with regard to vitamin D and fracture risk and BMD. The research suggests that Ca plays a major role in explaining changes in fracture risk and BMD changes; the actual independent role of vitamin D on bone health still needs to be elucidated. Also the meta-analysis by Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Willett and Wong63) did not include several important RCT completed recently such as those by Porthouse et al. (Reference Porthouse, Cockayne and King65) and Grant et al. (Reference Grant, Avenell and Campbell66).
Another comprehensive meta-analysis undertaken by the Cochrane Collaboration found vitamin D in various forms by itself had no statistically significant effect on new fracture incidence (RR 1·02; 95 % CI 0·93, 1·11); however, vitamin D and Ca were significantly associated with reduced risk of non-vertebral (RR 0·87; 95 % CI 0·78, 0·97) and hip fracture incidence (RR 0·81; 95 % CI 0·68, 0·96)(Reference Avernell, Gillespie and Gillespie67). Again this study supports the use of Ca with vitamin D in fracture prevention; however, as in the meta-analysis by Tang et al. (Reference Tang, Eslick and Nowson64), vitamin D alone seems to have no significant effect. Differences in results between this analysis and the meta-analysis by Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Willett and Wong63) may be partially explained by differing inclusion criteria and the wide use of subgroup analysis in these studies in order to separately analyse the effects of higher doses of vitamin D on fracture risk and bone density.
A recent meta-analysis by Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Willett and Wong68) assessing the efficacy of vitamin D oral supplementation on non-vertebral fractures reported that pooling trials with a higher dose than 400 IU/d (10 μg/d) of vitamin D was associated with a 20 % reduction in non-vertebral fractures for individuals aged 65 years and over. This study was significant also for its inclusion of twelve double-blind RCT for non-vertebral fracture and eight RCT for hip fracture (total n 83 165) and also the finding that this effect was independent of Ca supplementation(Reference Bischoff-Ferrari, Willett and Wong68). This recent study provides support for Biscoff-Ferrari's earlier meta-analysis in that it provides strong evidence for higher-dose vitamin D supplementation being needed for fracture prevention (i.e. >800–1000 IU/d or 20–25 μg/d) and the independent role of vitamin D in fracture prevention.
Intervention studies of vitamin D's role in osteoporosis
Several intervention studies have been conducted recently. investigating the relationship between vitamin D and osteoporosis risk (for intervention trials, see Table 4)(Reference Porthouse, Cockayne and King65, Reference Grant, Avenell and Campbell66, Reference Larsen, Mosekilde and Foldspang69–Reference Meier, Woitge and Witte77). Two recent large, intervention trials have shown inverse associations between vitamin D supplementation and fracture risk(Reference Larsen, Mosekilde and Foldspang69, Reference Trivedi, Doll and Khaw71). A large study by Larsen et al. (Reference Larsen, Mosekilde and Foldspang69) showed that participants supplemented with 10 μg (400 IU) cholecalciferol and 1000 mg Ca over a 3-year period were observed to have a 16 % reduction in fracture risk compared with those undertaking a domestic environment improvement programme. The above study by Larsen et al. (Reference Larsen, Mosekilde and Foldspang69) had a number of limitations, however, as only relatively small doses of vitamin D3 were used and it was impossible to separate the individual effects of Ca and vitamin D on fracture risk. In order to overcome this, Trivedi et al. (Reference Trivedi, Doll and Khaw71) used a study design whereby vitamin D3 supplements only were given to participants in 4-monthly megadoses of 2500 μg (100 000 IU) and compared these with a placebo group over 5 years. Results showed that first fracture risk was reduced by 22 % in the intervention group, while fracture risk for common osteoporotic sites (hip, wrist and vertebrae) was reduced by 33 %(Reference Trivedi, Doll and Khaw71). Two trials using BMD as the endpoint, rather than fracture incidence, have also shown that vitamin D3 and Ca supplementation or the use of vitamin D3- and Ca-fortified food products were associated with an increase in BMD(Reference Moschonis and Manios72, Reference Meyer, Smedshaug and Kvaavik73) (see Table 4). Both of these studies had a number of weaknesses including the use of relatively low levels of supplemental and dietary vitamin D (7·5–12·5 μg/d) and small subject numbers. However, they do provide support for the use of vitamin D3 and Ca for bone loss prevention and the use of fortified dairy products by Moschonis & Manios(Reference Moschonis and Manios72) was particularly unique considering the role of other bone nutrients in dairy products in osteoporosis prevention.
Table 4 Recent intervention studies on effect of vitamin D on fracture risk or bone losses

BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; HR, hazard ratio; RR, relative risk.
Other studies have shown no relationships between vitamin D and fracture risk. Recently, five RCT have shown no relationship between vitamin D and fracture risk (see Table 4). For instance, Lyons et al. (Reference Lyons, Johansen and Brophy74) reported that 4-monthly 100 000 IU (2500 μg) vitamin D2 supplementation was not significantly effective at reducing fractures among a group of older institutionalised elderly over a 3-year follow up. A possible weakness of this study was the use of ergocalciferol (vitamin D2), which has a reportedly lower potency and a shorter duration of action compared with the cholecalciferol (vitamin D3) form, although research has also shown that ergocalciferol may be just as effective as cholecalciferol in raising serum 25(OH)D status, so this area remains controversial(Reference Armas, Hollis and Heaney78, Reference Rapuri, Gallager and Haynatzki79). In the Women's Health Initiative study, a 12 % decrease in hip fracture in females supplemented with 1000 mg Ca and 10 μg vitamin D3 over 7 years was reported, but this was not significant(Reference Jackson, Lacroix and Gass70). The relatively small doses of vitamin D3 used in this study were a weakness, with other factors such as lower fracture rate due to high personal Ca intake and hormone therapy use also possible contributors to the lack of any significant improvement(Reference Jackson, Lacroix and Gass70). Another large RCT by Porthouse et al. (Reference Porthouse, Cockayne and King65) also found no evidence that fracture was reduced in women taking 20 μg vitamin D3 and 1000 mg Ca. The results from these trials are surprising, as three of the RCT were conducted in the institutionalised elderly, who have in the past been seen as the population to most benefit from vitamin D supplementation. Also, several of the trials used relatively large doses of vitamin D, which have been associated with an improvement in studies such as those by Trivedi et al. (Reference Trivedi, Doll and Khaw71).
Vitamin D's role in the secondary prevention of fractures
Secondary prevention of fractures is an area frequently overlooked by researchers; however, it is a very important area considering that the risk of fractures increases exponentially following the first osteoporotic fracture(55). In the NoNOF (Nottingham Neck of Femur) study researchers reported that Ca and vitamin D (either vitamin D2 or D3) interventions for elderly women who had suffered a hip fracture increased BMD by up to 4·6 % over a 1-year follow up (see Table 4)(Reference Harwood, Sahota and Gaynor75). This study was unique as a result of the multiple interventions used and showed that oral vitamin D3 and Ca given daily were more beneficial at increasing BMD than injected megadoses of vitamin D2 with or without oral Ca(Reference Harwood, Sahota and Gaynor75). A second study by the Randomised Evaluation of Calcium oR vitamin D (RECORD) trial group(Reference Grant, Avenell and Campbell66), however, showed no significant differences in incidence in low trauma secondary fractures for elderly participants on vitamin D3, Ca and vitamin D3 or a placebo followed over 24–62 months (see Table 4). The RECORD trial was a larger study and was conducted over a longer period of follow up compared with the intervention by Harwood et al. (Reference Harwood, Sahota and Gaynor75). While both studies used the same dose of oral vitamin D3 (20 μg/d), direct comparison was difficult as both reported different outcomes (BMD v. fractures). However, the RECORD trial calls into question the clinical significance of small BMD increases on fracture risk; i.e. despite small increases in BMD does this correspond to a decreased risk of secondary fractures in the elderly? Studies assessing both secondary fracture incidence and BMD changes are needed to improve the evidence for vitamin D supplementation in this field.
Observational studies of vitamin D's role in osteoporosis
At least two large observational studies have also recently shown a relationship between 25(OH)D and fracture risk(Reference Schoor, Visser and Pluijm80, Reference Gerdhem, Ringsberg and Obrant81). In these studies serum 25(OH)D levels under 30 and 50 nmol/l were significantly associated with an increased risk of fractures in the Longitudinal Aging Study Amsterdam (LASA) (n 1311; hazard ratio 3·1; 95 % CI 1·4, 6·.9) and Osteoporosis Prospective Risk Assessment (OPRA) (n 986; hazard ratio 2·04; 95 % CI 1·04, 4·04) studies, respectively(Reference Schoor, Visser and Pluijm80, Reference Gerdhem, Ringsberg and Obrant81). Caution is needed when interpreting these results, as Gerdhem et al. (Reference Gerdhem, Ringsberg and Obrant81) notes that fracture risk in those participants with 25(OH)D under 30 nmol/l was likely to be in part due to high frailty in this group. However, with vitamin D's proposed role in muscle strength and fall prevention, it is still likely to be involved in the causal pathway.
Conversely, the observational OFELY (Os des Femmes de Lyon) study (n 669) with a long median follow up of 11·2 years did not report any significant differences in fracture incidence, bone turnover or BMD in healthy home-dwelling women when classified according to 25(OH)D status ( ≤ 50 v.>50 nmol/l and ≤ 75 v.>75 nmol/l(Reference Garnero, Munoz and Somay-Rendu82)). A possible contributing factor for the differences of findings in these studies could be that participants in the OFELY study had a mean age of 62 years, which was much younger than the mean ages of the participants in the OPRA and LASA studies. The long length of follow up for the OFELY study compared with the OPRA and LASA studies, however, provides strong evidence that 25(OH)D levels in healthy younger postmenopausal women may not be a strong determinant of fracture risk or bone turnover.
Summary of vitamin D's role in osteoporosis
Despite some strong evidence to support vitamin D's role in fracture and bone loss prevention, recent, well-designed intervention and observational studies have cast some doubt on this and thus the evidence remains inconsistent. This is particularly true for studies using vitamin D without Ca in order to tease out the individual role of vitamin D in bone health. While all the studies reported decreases in PTH in response to vitamin D supplementation, changes in other bone measures are inconsistent across studies. An overlooked area that may have the potential to explain some of the inconsistencies in results seen with the research to date is the prevalence of osteomalacia in the samples of participants studied. For example, it has been reported that up to one-third of postmenopausal women have a secondary cause of low BMD, which can include osteomalacia(Reference Maricic83). Thus participants with osteomalacia in studies are likely to benefit from vitamin D supplementation and may suffer fewer fractures and increases in BMD caused by improvements to osteomalacia rather than a protective effect of vitamin D for osteoporosis. Therefore studies with a higher proportion of participants with osteomalacia (which is rarely documented) may show increased effectiveness of a vitamin D intervention. Other gaps in our knowledge include the lack of men recruited, heavy emphasis on European and North American populations and the use of widely varying dosages of vitamin D, despite strong evidence supporting the use of at least 20 μg (800 IU) for beneficial effects(Reference Bischoff-Ferrari, Willett and Wong68, Reference Bischoff-Ferrari and Dawson-Hughes84). This last point is particularly important, especially given the results of the recent meta-analysis conducted by Biscoff-Ferrari et al. (Reference Bischoff-Ferrari, Willett and Wong68), reporting the importance of higher-dose vitamin D in antifracture efficacy. Despite these inconsistencies, Ca and vitamin D, when used together in adequate doses, seem to be associated with improved bone health, especially in elderly individuals.
Vitamin D and muscle strength
There has been much recent interest and research with regard to the role that vitamin D plays in muscle strength and function. This is a very important area, as poor muscle strength and function have a negative impact upon balance and body sway, which in turn increase the risk of falling(Reference Pfeifer, Bergerow and Minne85, Reference Bischoff-Ferrari, Conzelman and Stahelin86). Falls are a serious health concern as they are a major cause of fractures and morbidity in older adults, with data from the Australian Institute of Health and Welfare showing that falls resulted in an estimated 60 497 hospitalisations in 2003–4 for individuals over 65 years of age in Australia(Reference Bradley and Harrison87).
One of the main pathways by which vitamin D is thought to affect muscle function is through the binding of 1,25(OH)2D to nuclear receptors on skeletal muscle; this stimulates, via gene transcription, protein synthesis which in turn has an impact on muscle growth(Reference Pfeifer, Bergerow and Minne85, Reference Bradley and Harrison87, Reference Bischoff-Ferrari88). Thus, vitamin D's role in reducing fracture risk may be explained not only by its role in maintaining BMD and making it less likely that bones will fracture when a fall occurs, but also by actually reducing the risk of a fall in the first place via 1,25(OH)2D's role in maintaining muscle strength and balance. This role of 1,25(OH)2D on skeletal muscle strength has been supported by a number of studies that have directly tested the muscle strength of participants, as well as fall and balance studies which have shown significant relationship between lower serum 25(OH)D status and poorer muscle strength and increased fall risk.
Vitamin D and studies directly measuring muscle strength
Two large investigations that have directly tested the muscle strength of participants are the OPRA study and the recent InChianti study(Reference Gerdhem, Ringsberg and Obrant81, Reference Houston, Cesari and Ferruci89). Both of these studies showed statistically significant associations with serum 25(OH)D and handgrip strength at the multivariate level (men P = 0·04; women P = 0·01) and correlations of 25(OH)D with thigh muscle strength (Spearman's rank test r 0·08; P = 0·02) for the InChianti and OPRA studies, respectively(Reference Gerdhem, Ringsberg and Obrant81, Reference Houston, Cesari and Ferruci89).
These studies had large samples of randomly selected participants, the OPRA study using a sample of 986 elderly women and the InChianti study using 976 males and females participants over 65 years and as such have provided strong evidence for the role of serum 25(OH)D in muscle strength(Reference Gerdhem, Ringsberg and Obrant81, Reference Houston, Cesari and Ferruci89). Similarly, in a large USA-based survey (n 4100) of individuals over age 60 years, Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Dietrich and Orav90) found that levels of serum 25(OH)D under 40 nmol/l were associated with decreased muscular function in the lower extremities. Other cross-sectional and cohort studies have also shown this trend(Reference Bischoff-Ferrari, Stahelin and Urscheler91–Reference Glerup, Mikkelsen and Poulsen93); however, one of these(Reference Bischoff-Ferrari, Stahelin and Urscheler91) only showed a correlation between 25(OH)D and muscle strength in males while the active 1,25(OH)2D form was correlated with muscle strength in both females and males.
However, not all studies have shown a relationship between muscle strength and serum 25(OH)D. Verreault et al. (Reference Verreault, Sembra and Volpato94) conducted a prospective cohort study with a 3-year follow up in older, disabled women (n 628) which showed no relationship in muscle strength, walking speed or repeated chair stands for groups stratified by serum 25(OH)D status ( ≥ 53 nmol/l, 25–52 nmol/l, ≤ 5 nmol/l). Despite a high-quality observation design, however, this study only measured moderately to severely disabled older women whose physical function was more than likely below baseline, so its applicability to ambulatory or even non-disabled elderly is severely limited. One of the major limitations of all of the above observational studies is, of course, their inability to infer a causal relationship between muscle strength and serum 25(OH)D due to their non-experimental design. Two recent RCT, however, have found no relationship between muscle strength measures and vitamin D(Reference Latham, Anderson and Lee95, Reference Kenny, Biskup and Robbins96). Kenny et al. (Reference Kenny, Biskup and Robbins96) provided 1000 IU (25 μg) vitamin D3 supplementation over 6 months to a group of ambulatory older men (aged 65+years) and provided a placebo to the control (total n 65). Handgrip strength, leg extension and a short physical performance battery was used to test muscle strength at baseline and following 6 months and while there was a significant increase in 25(OH)D status in the intervention group, no significant changes were seen in physical performance or muscle strength. Latham et al. (Reference Latham, Anderson and Lee95) used a larger sample of older frail elderly (n 243) and supplemented the intervention group with a single 300 000 IU (7500 μg) dose of calciferol at the beginning of the trial. Physical performance indicators of leg extension, Berg balance test, timed up and go test and time to walk a 4 m distance were tested at baseline, 3 months and 6 months(Reference Latham, Anderson and Lee95). At 6 months, calciferol supplementation had no significant impact on functional performance in these participants. Both these studies were well-designed RCT and together seem to indicate vitamin D supplementation had no effect on either healthy ambulatory men or the older frail elderly. However, the results need to be interpreted with caution, as relatively small numbers were used in both studies and only a small subgroup within the samples were classified as serum 25(OH)D deficient, while, in comparison, the larger InChianti observational study had a much larger number of serum 25(OH)D-deficient participants available for analysis. It is therefore likely that only vitamin D-deficient groups may benefit from supplementation.
In the future, areas of research may include a greater emphasis for the role of raised PTH levels, which have been linked independently with lower muscle strength, resulting in both low vitamin D and the related secondary hyperparathyroidism causing a loss of functional ability and balance(Reference Pfeifer, Bergeow and Minne97). The measurement of 1,25(OH)2D levels and its association with muscle strength may also be an area of some promise, as the study by Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Stahelin and Urscheler91) showed that this was correlated with muscle strength in both males and females.
Recent research has also focused on the role of VDR polymorphisms on muscle strength and fall risk(Reference Bischoff-Ferrari88, Reference Houston, Cesari and Ferruci89). Windelinckx et al. (Reference Windelinckx, De Mars and Beunen98) used genotyping in 493 men and women to show that various polymorphisms were associated with quadriceps strength in both men and women.
Vitamin D and fall risk
The main health outcome of reduced muscle strength and function is an increase in fall risk due to decreased balance and increased body sway. As a result of this, studies investigating the relationship between serum 25(OH)D and falls are more numerous than those that have directly investigated muscle strength and many of these studies have shown significant relationships between vitamin D supplementation or serum 25(OH)D status and reduced fall risk(Reference Dukas, Bischoff and Lindpaintner99–Reference Bischoff, Stähelin and Dick104). In a meta-analysis of five RCT, Bischoff-Ferrari et al. (Reference Bischoff-Ferrari, Dawson-Hughes and Willett105) reported that vitamin D supplementation with vitamin D3, calcitriol or calcidiol reduced the risks of falling by 22 % (corrected OR 0·78; 95 % CI 0·64, 0·92). Another meta-analysis by Jackson et al. (Reference Jackson, Gaugris and Sen106), which analysed five studies (four RCT and one prospective observational study), reported a pooled RR of 0·88 (95 % CI 0·78, 1·00) for vitamin D3 reducing falls. These meta-analyses included total sample sizes of 1237 and 1885 (both institutionalised and ambulatory) adults, respectively, and provide strong evidence for the role of vitamin D in reducing the risk of falls in both institutionalised and ambulatory adult populations.
A number of studies have not found a relationship between fall risk and serum 25(OH)D levels or vitamin D supplementation. RCT conducted by Dawson-Hughes et al. (Reference Dawson-Hughes, Harris and Krall107) and Chapuy et al. (Reference Chapuy, Pamphile and Paris108) both showed no significant effect on falls for vitamin D3 supplementation of 700 and 800 IU (17·5 and 20 μg) over 3 and 2 years, respectively. This was despite significant increases in serum 25(OH)D status in both studies. Thus, despite some strong evidence showing a reduction in fall risk for individuals with higher serum 25(OH)D status, there remains numerous inconsistencies in the evidence base. Large, well-designed RCT are really needed to overcome these inconsistencies, as many of the studies have relied on small numbers of participants, which affects the generalisibility of the results.
Summary of vitamin D and muscle strength
Vitamin D's role in muscle strength and fall risk is a relatively new area and thus many gaps exist in our knowledge. For instance, many of these trials also used supplemental Ca due to the proposed effect of both Ca and vitamin D on falls, but few used vitamin D and Ca supplementation and compared it with vitamin D alone. Dukas et al. (Reference Dukas, Bischoff and Lindpaintner99), however, showed that only those with a Ca intake above 512 mg/d had a significant reduction in fall risk with alfacalcidol supplementation, suggesting that this may be an important area of research. A number of studies also compared Ca v. Ca and vitamin D, showing vitamin D2 or vitamin D3 and Ca to be significantly more effective in reducing falls than Ca alone(Reference Pfeifer, Bergeow and Minne97, Reference Broe, Chen and Weinberg103). Also, few studies with regard to muscle strength and fall risk have been conducted in an Australian setting, which is disappointing when considering the enormous health burden that falls and osteoporosis places on this country.
Vitamin D and cancer
Vitamin D's possible role in the prevention of some types of cancer is one of the most important areas of research involving vitamin D, due to the enormous health impact of cancer worldwide. Research suggesting that vitamin D had a role in cancer can be traced back to an ecological study by Apperly(Reference Apperly109) which initially linked UV radiation exposure with cancer incidence in the USA. Although the link between vitamin D, UV exposure and cancer was not directly made, this landmark study showed that people in northern US states with low UV exposure such as New Hampshire were more likely to die of cancer than those living in southern states such as Texas with high UV exposure(Reference Holick110). It was only through later studies, however, that vitamin D was linked as a possible factor in reducing cancer risk(Reference Garland, Garland and Gorman111).
A variety of possible anti-carcinogenic biological mechanisms have been proposed to explain vitamin D's role in reducing cancer risk. 1,25(OH)2D (the active vitamin D form) is involved, via its nuclear VDR, in the regulation of cell differentiation, cell growth, apoptosis (cell death) and various cellular mechanisms that play a role in cancer development(Reference Ingraham, Bragdon and Nohe53). For instance, 1,25(OH)2D has been shown to be active in cell-cycle control via p21, p23 and p53 proteins, which block DNA-damaged cells progressing in the cell development cycle(Reference Ingraham, Bragdon and Nohe53). Also, it has been proposed that 1,25(OH)2D has a role in inducing apoptosis and inhibiting angiogenesis once a cell becomes malignant, therefore reducing the ability of the malignant cell to survive(Reference Holick47). Importantly colon, prostate and mammary cells have demonstrated the ability to extrarenally synthesise 1,25(OH)2D, which may play a role in modulating cell proliferation and apoptosis at the local level(Reference Lechner, Kallay and Cross112).
The variety of biological mechanisms in which 1,25(OH)2D can affect cancer development, shown by experimental in vitro studies, provide a good biological basis for the protective role of vitamin D in cancer. However, there is obviously a need for well-designed, population-based epidemiological studies to provide evidence of this association. The main types of cancer that vitamin D has been linked to are: colorectal, breast and prostate; however, links with other types of cancer such as lung, ovarian and endometrial have also been suggested(Reference Garland, Garland and Gorman111).
Prostate cancer
Studies investigating the relationship between vitamin D and prostate cancer have produced contradictory evidence(Reference Touhimaa, Tenkanen and Ahonen113). Hanchette & Schwarz(Reference Hanchette and Schwarz114) showed a highly significant (P ≤ 0·0001) inverse relationship between UV exposure and prostate cancer risk in a large US ecological study and hypothesised that the high prostate cancer risk in those areas with lower UV exposure was due to individuals' lower vitamin D levels. Even at the time of this study (1992), the possible anti-tumour effects of vitamin D were well known and the presence of VDR on prostate cells indicated a role for vitamin D in the regulation of prostate cell growth(Reference Berger, Wilson and McCleelland115). However, ecological studies have many weaknesses, including a very high risk of confounding, so further research using higher-quality research designs was warranted to test this hypothesis.
A number of recent case–control designs have found associations between serum 25(OH)D and prostate cancer(Reference Ahonen, Tankanen and Teppo116–Reference Platz, Leitzmann and Hollis118). Ahonen et al. (Reference Ahonen, Tankanen and Teppo116) showed that men aged under 52 years with serum 25(OH)D levels below 40 nmol/l were 3·5 times more likely to be diagnosed with prostate cancer (OR 3·5; 95% CI 1·7, 7·0; P < 0·0006) while men aged over 52 years, with the same low levels of serum 25(OH)D, had almost no increase in risk (OR 1·2; 0·7 ± 2·1; P not reported). One possible reason proposed by the authors for this is that vitamin D may inhibit androgen-induced prostate cell proliferation and men aged over 50 years have increasingly lower androgen activity; therefore vitamin D has a much reduced effect on prostate cancer risk in older men(Reference Ahonen, Tankanen and Teppo116). A second larger case–control study interestingly showed a U-shaped association, whereby groups of participants who had serum 25(OH)D levels of < 19 nmol/l and >80 nmol/l showed an increased risk of prostate cancer (OR 1·5 (95 % CI 0·8, 2·7) and OR 1·7 (95 % CI 1·1, 2·4)), respectively(Reference Touhimaa, Tenkanen and Ahonen113). The authors hypothesised that the group with higher serum 25(OH)D may have experienced a higher risk of prostate cancer due to higher intakes of vitamin A or by increased activation of 24-hydroxylase (an enzyme which inactivates vitamin D), leading to local vitamin D deficiency in the prostate gland(Reference Touhimaa, Tenkanen and Ahonen113). Studies by Gann et al. (Reference Gann, Ma and Hennekens117) and Platz et al. (Reference Platz, Leitzmann and Hollis118), however, have shown no relationship between either 1,25(OH)2D or 25(OH)D and prostate cancer, although both of these studies did not rule out small to moderate effects or effects at later disease stages, respectively.
All of these studies suffer limitations due to their case–control design, so we cannot infer any causal relationship. Also geographical differences (Scandinavian studies tended to have lower mean and median serum 25(OH)D), season of blood draws, control matching and follow-up time all make it difficult to form a consistent picture. Further research using higher-quality research designs such as large cohort studies are needed to better determine the relationship between vitamin D and prostate cancer.
Breast cancer
Vitamin D's role in breast cancer aetiology has been one of the major areas of vitamin D and cancer research. This has been due, in part at least, to ecological studies showing geographical differences in breast cancer mortality or incidence that were closely associated with differences in UV exposure(Reference Gann, Ma and Hennekens117). Experimental evidence has provided some strong support for the hypothesis that vitamin D may have a protective role against breast cancer. In vitro studies have shown that 1,25(OH)2D can stimulate apoptosis, cell-cycle arrest and reduce oestrogen receptor expression in MCF-7 breast cells(Reference Rohan119).
Two epidemiological studies by Lowe et al. (Reference Lowe, Guy and Mansi120) and Bertone-Johnson et al. (Reference Bertone-Johnson, Chen and Holick121) have investigated circulatory 25(OH)D levels and breast cancer risk. Lowe et al. (Reference Lowe, Guy and Mansi120) recruited hospital breast cancer patients (n 179) and controls (n 170) and reported that women with 25(OH)D levels < 50 nmol/l had an OR of 3·54 (95 % CI 1·89, 6·61; P < 0·001) compared with those women with 25(OH)D levels>50 nmol/l. In a larger study by Bertone-Johnson et al. (Reference Bertone-Johnson, Chen and Holick121) (701 cases and 724 controls), women with the highest quintile of 25(OH)D had a 27 % decreased risk of breast cancer compared with those in the lowest quintile (RR 0·73; 95 % CI 0·49, 1·07; P trend = 0·06). 1,25(OH)2D was also tested in this study but non-significant results were found. Both of these studies provide evidence for an inverse association of higher serum 25(OH)D with breast cancer risk, although a weakness of both of these studies was that serum 25(OH)D was tested only once and not repeated, which may not reflect long-term serum 25(OH)D status. It could also be argued that serum 25(OH)D status may not accurately reflect the local concentration of the active form of vitamin D, 1,25(OH)2D, which has been shown in vitro to mediate anti-cancer effects in breast cells (see above). Lowe et al. (Reference Lowe, Guy and Mansi120) comments on this by postulating that adequate serum 25(OH)D is needed to provide a substrate for the 1α-hydroxylase enzyme in breast cells that produce the active 1,25(OH)2D locally.
A number of studies have also explored the relationship between vitamin D intake, sun exposure and risk of breast cancer. Cohort studies undertaken by Shin et al. (Reference Shin, Holmes and Hankinson122), McCullough et al. (Reference McCullough, Rodriguez and Diver123), John et al. (Reference John, Schwartz and Dreon124) and Robien et al. (Reference Robien, Cutler and Lazovich125) all found some inverse associations between vitamin D intake and/or exposure and breast cancer risk. Shin et al. (Reference Shin, Holmes and Hankinson122), as part of the Nurses' Health Study (n 88 691), reported no significant associations between total (dietary and supplemental) vitamin D intake and breast cancer incidence in postmenopausal women but did report significant associations when comparing the lowest total vitamin D intake ( ≤ 3·75 μg) with the highest vitamin D intake (>12·5 μg) in premenopausal women (RR 0·72; 95 % CI 0·55, 0·94; P = 0·01). McCullough et al. (Reference McCullough, Rodriguez and Diver123) conducted another study into a large cohort (n 68 567) as part of the Cancer Prevention Study II Nutrition Cohort and showed a statistically significant relationship between oestrogen receptor-positive tumours and dietary vitamin D intake (RR 0·74; 95 % CI 0·57, 0·93; P = 0·006), but overall breast cancer risk was not associated with vitamin D intake. Robien et al. (Reference Robien, Cutler and Lazovich125) reported a weaker non-statistically significant relationship with a RR of 0·89 (95 % CI 0·77, 1·03; P = 0·12) for women with a vitamin D intake of ≥ 20 μg in the Iowa Women's Health Study. All of these studies are notable due to their large size and prospective cohort design; however, as these were observational studies a causal relationship cannot be confirmed and both studies did not adequately measure sun exposure, which is the main source of vitamin D for most populations. John et al. (Reference John, Schwartz and Dreon124) in the First National Health and Nutrition Examination Survey (NHANES I) study (n 5009), however, did measure sun exposure via interview as well as dietary and supplemental intake of vitamin D. While this study did not show overall that vitamin D was associated with breast cancer reduction, it did infer that vitamin D intake ( ≥ 5 μg/d) and high solar radiation were associated with a reduction in risk (RR 0·71; 95 % CI 0·44, 1·14); however, this was not statistically significant(Reference John, Schwartz and Dreon124). Two major limitations of this study were the relatively small number of breast cancer cases detected, which limited statistical power and the use of 24 h dietary recall methods, which rarely represent participants' usual dietary intake.
A recent high-quality, double-blind, placebo-controlled RCT conducted by Lappe et al. (Reference Lappe, Travers-Gustafson and Davies126) has provided a boost to evidence of vitamin D's role in breast cancer reduction. While the primary outcome of this study was fracture incidence, a secondary outcome was cancer incidence. This study was conducted over a 4-year period with a randomly selected population of 1179 community-dwelling women aged>55 years(Reference Lappe, Travers-Gustafson and Davies126). A significant relationship between lower breast cancer risk in the Ca and vitamin D3 supplementation group (1400–1500 mg/d and 27·5 μg/d, respectively) compared with the placebo group (RR 0·402; 95 % CI 0·2, 0·82; P = 0·013) was reported. Furthermore, Ca plus cholecalciferol proved more effective at reducing the risk of breast cancer than Ca alone(Reference Lappe, Travers-Gustafson and Davies126).
There has also been much recent interest in the role of VDR polymorphisms such as Bsm1, Apa1, Taq1 and Poly(A) and breast cancer risk; however, studies into these have produced highly inconsistent results, probably related to methodological issues such as small sample sizes(Reference Rohan119). So, despite some promising experimental and epidemiological results, vitamin D's role in breast cancer prevention is still an area of some debate and further well-designed intervention studies, such as that undertaken by Lappe et al. (Reference Lappe, Travers-Gustafson and Davies126), and observational studies that use validated tools to collect sun exposure and dietary vitamin D data are needed to strengthen the evidence base.
Colorectal cancer
As was the case for prostate and breast cancer, the possible protective role of vitamin D against colorectal cancer was first proposed in a landmark ecological study conducted by Garland & Garland(Reference Garland and Garland127), which showed an inverse relationship between UV exposure and colorectal cancer risk. Since then, experimental data have shown that 1,25(OH)2D may be involved in controlling cell growth and differentiation in colon cells and the ability of the colon to convert 25(OH)D to active 1,25(OH)2D via local 1α-hydroxylase activity(Reference Lechner, Kallay and Cross112).
A meta-analysis of studies that have assessed the association between serum 25(OH)D status and colorectal cancer was conducted by Gorham et al. (Reference Gorham, Garland and Garland128). This analysis identified five nested case–control studies (total n 1448) using pre-diagnostic serum from healthy volunteers who were followed up between 2 and 25 years for incidence of colorectal cancer. Results from the pooled analysis of this study showed that serum 25(OH)D levels ≥ 83 nmol/l were associated with a 50 % lower risk of colorectal cancer compared with serum 25(OH)D levels < 30 nmol/l(Reference Gorham, Garland and Garland128). A number of studies have also shown a relationship between dietary vitamin D intake and colorectal cancer risk, including a systematic review by Gorham et al. (Reference Gorham, Garland and Garland129), which included analysis of fourteen epidemiological studies investigating vitamin D intake and colorectal cancer. The authors summarised that vitamin D intakes of ≥ 25 μg/d were associated with a decrease in colon cancer risk of approximately 50 % compared with vitamin D intakes of < 2·5 μg/d(Reference Gorham, Garland and Garland129). It should be noted, however, that groups with high or moderate sun exposure and displaying adequate to optimal serum 25(OH)D levels, even with low vitamin D dietary intakes, would almost certainly not obtain the same benefits as described above. This is supported by the fact that many of the studies supporting the role of oral vitamin D intake and colon cancer prevention are conducted in high latitude northern countries displaying low baseline serum 25(OH)D levels and are therefore most likely to benefit from increased oral vitamin D intake(Reference Gorham, Garland and Garland128). Other studies have shown a null relationship; for instance, the recent, large Women's Health Initiative (n 36 282) RCT reported no statistically significant difference in colorectal cancer risk in women receiving both Ca (1000 mg/d) and vitamin D3 (10 μg/d) supplements compared with those receiving a placebo over a 7-year follow up(Reference Wactawski-Wende, Kotchen and Anderson130). One significant weakness of this study was its use of relatively low doses of vitamin D3, when other studies have found that doses of 25 μg/d may be needed to significantly reduce the risk of colon cancer(Reference Gorham, Garland and Garland129). Although causal inference cannot be inferred due to observational study designs, there is a growing body of evidence showing that vitamin D may reduce the risk of colorectal cancer. However, the data are not fully consistent with some large, well-designed studies such as that conducted by Wactawski-Wende et al. (Reference Wactawski-Wende, Kotchen and Anderson130) showing no association. A large well-designed RCT with similar methodology to the Women's Health Initiative, but using higher vitamin D doses (for example, 25 μg/d) and a longer follow up, is needed to establish a causal link between vitamin D and colorectal cancer risk.
Summary of vitamin D's role in cancer
Despite the strong experimental in vitro and ecological data that support a possible role for vitamin D in reducing cancer risk, epidemiological evidence is relatively inconsistent, at least for most types of cancer. The use of latitude gradients of disease based on erythemal UV levels, which has formed the basis of many of the ecological studies, has been recently called into serious question, with actual vitamin D-producing UV showing significant differences from erythemal UV based on latitudes(Reference Kimlin, Olds and Moore21). Ecological-based studies using geographical variations in UV exposure are also subject to a high degree of error due to major variations in individuals' UV exposure for each given area, so these studies need to be interpreted with caution. Also most of the studies showing a link between vitamin D and cancer are observational, so causal linkage cannot be inferred. There is also the alternative explanation, in light of the inconsistencies, that serum 25(OH)D status may not be linked to cancer. Observational designs are at high risk of confounding, so confounding factors such as obesity, which is linked both to increased cancer risk and low serum 25(OH)D status, may possibly explain some of the positive findings(Reference Aasheim, Hofso and Hjelmesaeth131).
There does, however, appear to be a growing body of in vitro and observational evidence supporting a role for vitamin D, especially in breast and colorectal cancers; large intervention trials are really needed to confirm causal linkages. Also, while much of the focus has been on vitamin D and breast, prostate and colorectal cancers, further research into the possible roles of vitamin D and other cancers such as lung and endometrial is needed.
Other proposed roles of vitamin D in disease prevention
Due to the widespread distribution and identification of VDR in many human tissue types, interest in vitamin D's role in the aetiology of other disease classes such as autoimmune and cardiovascular diseases has been of great interest to researchers. Other roles for vitamin D in disease aetiology continue to be found; for instance, a recent example of an antimicrobial role for vitamin D was demonstrated, with the finding of a VDR-mediated innate immune response against mycobacterium tuberculosis, resulting in bacterial killing(Reference Lui, Strenger and Li132).
Vitamin D and autoimmune diseases
VDR have been identified in dendritic cells, antigen-presenting cells, mononuclear cells and activated T and B lymphocytes(Reference Cutolo and Otsa133). Additionally, 1α-hydroxylase activity is expressed by activated macrophages and dendritic immune cells(Reference Baeke, Van Etten and Overbergh134). A number of possible mechanisms for the role 1,25(OH)2D in immune regulation have been identified; these include the down-regulation of Th1 cell activity, decreased release of cytokines from macrophages and B cell antibody production and reduced release of IL-2 and interferon-γ by CD4 T cells(Reference Cutolo, Otsa and Uprus135). A number of recent epidemiological studies have found associations between serum 25(OH)D status(Reference Kamen, Cooper and Bouali136, Reference Aguado, del Campo and Garces137) or total intake of dietary vitamin D via dietary and supplemental sources(Reference Munger, Zhang and O'Reilly138, Reference Hypponen, Reunanen and Jarvelin139) and common autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel diseases, systemic lupus erythematosis and type 1 diabetes mellitus(Reference Cantorna140). Of particular note, due to its large prospective cohort design, is the study conducted by Munger et al. (Reference Munger, Zhang and O'Reilly138) as part of the Nurses' Health Study I and II (92 253 women between 1980–2000 and 95 310 women between 1991–2001, respectively) in which dietary vitamin D intake was assessed against risk of multiple sclerosis in the two cohorts. Pooled analysis comparing women in the highest quintile of vitamin D intake with those in the lowest quintile revealed an age-adjusted RR of 0·67 (95 % CI 0·40, 1·.12; P = 0·03), suggesting a strong inverse association between total intake of vitamin D and multiple sclerosis(Reference Cantorna140). Other studies have suggested relationships between serum 25(OH)D status and the development of autoimmune disease(Reference Kamen, Cooper and Bouali136, Reference Aguado, del Campo and Garces137). While these results, combined with experimental models, are promising, a significant amount of our data is still sourced from ecological studies. More data using intervention trials are really needed to confirm whether higher serum 25(OH)D status is actually protective against certain autoimmune diseases, or that these positive associations are the result of confounding factors.
Vitamin D and cardiovascular diseases
Vitamin D's role in the aetiology of CVD is an area of great debate, as epidemiological studies have reported inconsistent results. Ecological studies such as that conducted by Zitterman et al. (Reference Zitterman, Schleithoff and Koerfer141) have shown higher CVD rates in higher latitudes and in winter months where sunlight exposure is less. This led to the hypothesis that vitamin D, the main beneficial outcome from sunlight exposure, is linked to CVD aetiology. As in cancer studies, ecological studies based on UV exposure by latitude or season are very limited, as large individual variations are commonplace. As a result of this, no firm causal links have been established as yet; however, vitamin D has also been linked inversely to risk factors for CVD events such as hypertension (via inhibition of the renin–angiotensin–aldosterone system), diabetes and inflammation(Reference Michos and Melamed142).
The recent Women's Health Initiative Randomized Trial, however, reported no association between women taking 5 μg (200 IU) vitamin D twice daily and CVD risk over a 7-year follow up(Reference Hsia, Heiss and Ren143). However, as discussed in other sections of the present paper, 10 μg (400 IU) vitamin D per d may not provide an adequate dose to produce a preventative effect on disease. Research into vitamin D's role in CVD is still a relatively new area and new large RCT with adequate doses of vitamin D and follow up are really needed to confirm a causal relationship; however, with the enormous burden of disease that CVD places on the population, this is an extremely important area of ongoing and future vitamin D research.
Conclusion
Vitamin D is unique among the micronutrients in that while it is described as a fat-soluble vitamin it is more accurately classified as a steroid hormone. This is due to its action via specific VDR and the fact that most of our requirements are sourced from non-dietary means via UV radiation from sunlight exposure. As a result of the wide distribution of VDR in human tissues, vitamin D's role in human health is also diverse, ranging from its widely known function in bone health to possible roles in the prevention of certain cancers.
When it is considered that most humans rely on UV radiation, a known carcinogen, to synthesise the vast majority of their vitamin D, this creates a major worldwide health dilemma. This dilemma is further enhanced when it is considered that vitamin D deficiency has been identified as a problem in a sunny country such as Australia, which is known as the skin cancer capital of the world. In countries that have lower sunlight exposure such as in Northern Europe, inadequate vitamin D is an even greater problem. Wider spread fortification, as seen in some countries, or supplementation may be the most practical measures to alleviate suboptimal vitamin D levels, at least in vulnerable populations.
Vitamin D deficiency and indeed suboptimal vitamin D status play a major and increasingly broad and complex role in health and disease. While vitamin D's functions in bone health are widely known, its importance is set to grow in this area, driven by ageing populations in Western societies which are contributing to ever-growing numbers of individuals suffering from osteoporosis. Furthermore, with the discovery of the widespread distribution of VDR and enzyme activity in various human tissues, biologically plausible mechanisms have appeared to support previous epidemiological evidence suggesting links with various diseases and conditions beyond bone health. These include roles in cancer, autoimmune disease, CVD and muscle function. Whilst there have been many promising findings for these ‘new’ areas of vitamin D research, much remains to be discovered and in some cases the epidemiological evidence as a whole has been inconclusive. Many of the designs used are observational and are therefore at high risk of confounding. This raises the possibility that some positive associations seen between serum 25(OH)D and disease may be influenced by confounding factors such as obesity and lifestyle. In conclusion, future research with an emphasis on high-quality observational and experimental designs is needed to further elucidate the role and potential public health impacts that vitamin D may offer for both traditionally associated roles such as bone health and more recent potential benefits for diseases such as cancer and CVD.
Acknowledgements
This project was funded through a scholarship from the Queensland University of Technology's School of Public Health. Both authors were equally involved in the research and preparation of this paper.
D. B. declares no conflicts of interest for either author.