The present review will seek to review the research on Cu and Mo metabolism within the rumen with relevance to thiomolybdate toxicity in ruminant animals. It will demonstrate that the production of thiomolybdates is a clinical problem and actually causes a thiomolybdate toxicity rather than inducing a Cu deficiency.
The problem of Fe, S and Mo interactions with Cu has been a contentious and confused issue in recent times(Reference Laven and Livesey1–Reference Telfer, Kendall and Illingworth4). Signs attributed to ‘clinical Cu deficiency’ in ruminants are usually, in the field, non-specific and ‘subclinical’ and include: reduced weight gain, decreased food intake, reduced efficiency of food conversion, alteration in hair/wool texture and pigmentation (spectacles around eyes), delayed puberty, reduced conception rate, inhibition of oestrus, swayback. These do not tend to be the contention, but whether they are caused by a ‘lock up’ of Cu (induced or secondary Cu deficiency) or by the toxic effects of thiomolybdates is. However, much of the historical research into this area has been overlooked, hence the requirement for the present review.
The review will be based on the mechanism outlined in Fig. 1 and each stage will be examined individually in order to elucidate the mechanism. Briefly, Mo, S (as sulfate, sulfide or S amino acids), Cu and Fe all enter the rumen via feed, soil, water or supplements. Reactions occur between Mo and S enabling the formation of thiomolybdate compounds which will readily bind Cu. In the absence of rumen-available Cu (rumen-labile Cu), thiomolybdates are able to be absorbed through the rumen wall and small intestine, allowing them to bind to Cu-containing substances, including enzymes whose activity will be reduced, thus causing clinical problems often quoted as Cu deficiency, even though it is strictly a thiomolybdate toxicity. Another interaction between Fe, S and Cu will intensify the thiomolybdate problem by making Cu unavailable to bind to the thiomolybdates. In the absence of Mo and Fe interactions, the absolute dietary requirement for Cu is very low; experimentally this has been shown to be < 1·6 mg/kg DM(Reference Moeini5).
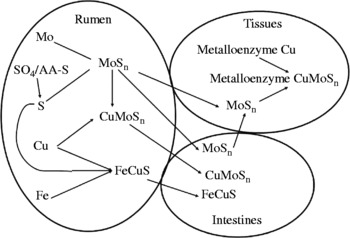
Fig. 1 Ruminal mechanism for the interaction of Cu, Mo, S and Fe and routes of absorption for the interaction products. MoSn is used diagrammatically to represent the thiomolybdate series (![]() , where n is 1 to 4).
, where n is 1 to 4).
What does TCA-soluble and -insoluble copper measure?
Much of the literature discussed within the present review uses TCA-insoluble fractions as an experimental result. In order to understand this, we need to appreciate what the TCA precipitation is measuring. The TCA precipitation method involves the addition of equal volumes of TCA solution (5–10 %) to the sample, the precipitate is then removed by filtration or centrifugation and the liquid fraction can be measured by the same methodology as the unprecipitated sample. The TCA-insoluble fraction can either be assessed by difference or by digestion of the precipitate/filtrate.
As early as 1975, Dick et al. (Reference Dick, Dewey and Gawthorne6) showed that the in vitro addition of increasing amounts of di- (![]() ), tri- (MoS3O2 −), or tetra-thiomolybdate (
), tri- (MoS3O2 −), or tetra-thiomolybdate (![]() ), to plasma (which was derived as the filtrate fraction from previous TCA precipitation) resulted in decreased Cu within the TCA-soluble filtrate until at approximately 3 μg/ml plasma all of the Cu was found in the TCA precipitate. In vitro addition of molybdate with or without sulfate had no effect on the Cu in the TCA precipitate. In the absence of thiomolybdates, the Cu was in the TCA-soluble fraction with Cu in the TCA precipitate being negligible. This suggested that TCA-insoluble Cu can be used as an indirect measure of thiomolybdate, differentiating thiomolybdate effects from Mo and S in non-thiomolybdate form.
), to plasma (which was derived as the filtrate fraction from previous TCA precipitation) resulted in decreased Cu within the TCA-soluble filtrate until at approximately 3 μg/ml plasma all of the Cu was found in the TCA precipitate. In vitro addition of molybdate with or without sulfate had no effect on the Cu in the TCA precipitate. In the absence of thiomolybdates, the Cu was in the TCA-soluble fraction with Cu in the TCA precipitate being negligible. This suggested that TCA-insoluble Cu can be used as an indirect measure of thiomolybdate, differentiating thiomolybdate effects from Mo and S in non-thiomolybdate form.
Are thiomolybdates produced in the rumen?
Dick et al. (Reference Dick, Dewey and Gawthorne6) showed that duodenal administration of thiomolybdates increased the Cu content in the TCA precipitate of blood plasma, whereas a duodenal infusion of molybdate (with or without S) had no effect whatsoever on the blood plasma TCA-insoluble Cu. This study indicates but does not prove that the thiomolybdate must be absorbed to affect the TCA-insoluble Cu in plasma and also that thiomolybdates are not formed in the duodenum. A suspension of rumen micro-organisms was incubated in a solution containing molybdate and sulfate; this resulted in the production of a mixture of di-, tri- and tetra-thiomolybdates which were identified by their absorption spectra(Reference Dick, Dewey and Gawthorne6). This led to Dick et al. (Reference Dick, Dewey and Gawthorne6) suggesting the following mechanism: sulfates are first reduced to sulfides by ruminal micro-organisms, the sulfide then reacts with the molybdate to form the thiomolybdates, the thiomolybdates then react with Cu to form insoluble Cu thiomolybdates, thus limiting the absorption of dietary Cu. In the field, situations with high dietary or water sulfides often have exacerbated ‘copper’ problems. The reduction of sulfate to sulfide in the proposed mechanism explains the increased potency found with increased dietary or water sulfide intakes.
Bray et al. (Reference Bray, Suttle and Field7) found tri- and tetra-thiomolybdates to be the predominant species found in cultured rumen fluid. Using non-fermentation ruminal conditions, Clarke & Laurie(Reference Clarke and Laurie8) showed the formation of the relative thiomolybdates to be pH- and S (given as sulfide):Mo molar ratio-dependent. Clarke & Laurie(Reference Clarke and Laurie8) showed at pH 7·0 that molar S:Mo ratios of < 3 only produced mono-thiomolybdates, 5–10:1 resulted in di-thiomolybdates, above 10 quickly transformed to tri-thiomolybdates, but found total conversion to tetra-thiomolybdate occurring at about 300:1. An S:Mo molar ratio of 62:1 at pH 8·0 resulted in the di-thiomolybdate being the predominant form (with no significant tetra-thiomolybdate), whereas at pH 6·5 the tri form dominated, and as pH decreased the tetra-thiomolybdate form increased. The modern dairy cow has a much lower ruminal pH than the older-type hay-fed dairy cow. They also reported S:Mo ratios found in the field and in supplementation trials to range from 6:1 up to 8000:1, suggesting that many field conditions would produce mainly tetra-thiomolybdates. Bray et al. (Reference Bray, Suttle and Field9) showed that the actual thiomolybdate production in rumen fermentation conditions, especially the production of tetra-thiomolybdate, was much greater than the Clarke & Laurie(Reference Clarke and Laurie8) work predicted. A third of molybdate was found to be converted to tetra-thiomolybdate over a wide range (4–12 μg Mo per ml) of Mo inputs and at S:Mo molar ratios of < 10. The molar ratios of S:Mo required for production of the tetra-thiomolybdate in this fermentative culture system were much lower than Clarke & Laurie(Reference Clarke and Laurie8) found for their non-fermentation culture. The system used by Clarke & Laurie(Reference Clarke and Laurie8) was also a closed system with no removal or binding of the product produced and was therefore allowed to come to equilibrium.
Incubations of sieved (through a 0·9 × 0·9 mm muslin mesh) rumen fluid (from cattle fed no Mo) with molybdate (0·1 mm) and sulfate (2·4 mm) by Elgallad et al. (Reference Elgallad, Mills and Bremner10) resulted in the production of an absorption spectra indicative of tetra-thiomolybdate (239, 317 and 467 nm) with no peaks indicative of di- or tri-thiomolybdates (392 and 394 nm), indicating that only tetra-thiomolybdate was produced in significant amounts. However, when Cu was added to the culture before the incubation there was no evidence of tetra-thiomolybdates in the rumen fluid culture. Transferring their work into the whole animal, Elgallad et al. (Reference Elgallad, Mills and Bremner10) showed that in cattle fed 100 mg Mo per kg DM, 3 g S per kg DM and 200 mg Cu per kg DM, there was no TCA-insoluble Cu and little albumin-bound Cu. However, when Cu was reduced to 1 mg/kg DM the TCA-insoluble Cu content increased and greater than 80 % of this was associated with albumin, Mo and Cu; the caeruloplasmin concentrations were also reduced. When a Cu injection was given to these low-Cu (1 mg/kg DM)-fed cattle, the plasma Cu increased, as did the plasma Mo, and both the total amount and proportion of Cu in the TCA-insoluble fraction were increased.
The mechanism by which the thiomolybdates are formed is a stepwise dehydrolysis of molybdate with an O being replaced by an S from a sulfide donor at each stage, the O being released as a water molecule(Reference Harmer and Sykes11). This reaction is reversible and equilibria are temperature and pH dependent. The formation is outlined in Fig. 2.

Fig. 2 Reactions that occur to form thiomolybdate in the rumen. The reactions are reversible. The direction is driven by the availability of molybdate and sulfide and the removal of the formed thiomolybdates by absorption and binding to the solid phase of forming the copper thiomolybdate complex within the rumen.
The study by Price et al. (Reference Price, Will and Paschaleris12) in which a large dose of 99Mo-labelled tetra-thiomolybdate was administered intra-ruminally and 99Mo-labelled tri- and di-thiomolybdates were recovered from the rumen digesta indicated the reversibility of this reaction in the rumen.
Whether the thiomolybdates are associated with the liquid or the solid phase of the rumen digesta is also of importance, as association with the solid phase imparts some stability on the thiomolybdates formed in the rumen, whereas the thiomolybdate in the liquid phase when remaining unbound, or unabsorbed is readily hydrolysed, reversing the reaction outlined above(Reference Price, Will and Paschaleris12). Available Cu within the digesta is also largely associated with the solid phase(Reference Price, Will and Paschaleris12), therefore potentially facilitating the intraruminal formation of Cu–thiomolybdate complexes.
It is doubtful that the equilibria are reached under rumen conditions due to the availability of the substrates, molybdate and S (as sulfide, or after rumen bug conversion to sulfide), along with the constant removal of the tetra-thiomolybdate. Therefore the reaction is most probably driven to the constant formation of thiomolybdates. The tetra-thiomolybdate would seem to have the following potential fates: (1) absorbed through the rumen wall; (2) passed with digesta through to the abomasum; (3) intra-ruminally bound to Cu, forming insoluble copper thiomolybdates which are egested; (4) bound to the solid phase which seems to incur some chemical stability; (5) hydrolysed in the liquid phase (i.e. involved in the equilibria). This makes this a very dynamic system.
Do thiomolybdates transfer into the animal and how?
Kelleher et al. (Reference Kelleher, Ivan and Lamand13) showed that 99Mo-radiolabelled tetra- and tri-thiomolybdates administered directly into the rumen of sheep by stomach tube appear within the plasma within 5 min, mainly in a TCA-insoluble form. However, when 99Mo-radiolabelled molybdate was administered by the same route, only small traces were found in the plasma over the first 60 min, with these predominantly in the TCA-soluble plasma fraction; after 2 h the amounts of 99Mo in the plasma increased rapidly and from 4 h the radiolabel was predominantly in the TCA-insoluble fraction, suggesting that appearance of the label into the TCA-soluble or -insoluble fraction is rate dependent – rapid uptake from a large ruminal thiomolybdate dose gives a large insoluble TCA fraction, whereas a slower digestive trickle feed gives more in the TCA-soluble fraction. Further, this indicates that more of the thiomolybdate from the large dose appears in plasma in a non-protein-bound form, whereas the trickle dose results in a greater proportion of the thiomolybdate being protein bound. The trickle dose of thiomolybdates gives ‘free’ or ‘rumen-available’ Cu much more opportunity to ‘detoxify’ the thiomolybdates. It must be remembered that the above effects are ruminal, as these sheep had duodenal re-entrant cannulae fitted, which allowed the digestion and absorption from the rumen to be totally separate from the intestinal digestion and absorption, as digesta was exchanged between the rumen output of one sheep and the duodenal input of the partner sheep and vice versa. Initially after starting to receive the exchange digesta, the proportion of TCA-soluble 99Mo in plasma for both tetra- and tri-thiomolybdate for the rumen administered/duodenal absorbed sheep was increased in comparison with the rumen administered/rumen absorbed partner (first 80 h), but the TCA-insoluble fraction was similar after 30 h. For the molybdate-administered sheep the digesta-exchanged sheep had virtually entirely TCA-soluble 99Mo for the first 4 h, but between 4 and 6 h the TCA-insoluble fraction increased rapidly but to only half of the level of the TCA-soluble fraction. The TCA-soluble fraction declined more rapidly than the TCA-insoluble fraction.
Hynes et al. (Reference Hynes, Woods and Poole14) showed, using 99Mo-labelled molybdate administered to cattle, that there was maximal plasma concentration of labelled Mo after 20 h, although similar to Kelleher et al. (Reference Kelleher, Ivan and Lamand13) this was initially mainly in the TCA-soluble fraction and the TCA-insoluble fraction lagged behind but remained more persistent as the TCA-soluble labelled Mo declined. Hynes et al. (Reference Hynes, Woods and Poole14) also showed that protein-bound 99Mo-labelled compounds were present. They eluted this protein with unlabelled tetra-thiomolybdate and displaced significant proportions of di- and tri-thiomolybdate and trace amounts of molybdate. They were unable to elute any tetra-thiomolybdate using this technique; however, whether this was as none was present or that unlabelled tetra-thiomolybdate was unable to displace the labelled tetra-thiomolybdate (both would have similar affinities) remains unclear. Mason et al. (Reference Mason, Lamand and Kelleher15) showed similar effects in sheep with no apparent production of tetra-thiomolybdate; however, they used the same elution technique and no account was made of the Cu content of the animal or diet.
In steers either given or not given a Cu injection immediately before an injection of radiolabelled tri-thiomolybdate, the steers with the Cu injection had virtually all of the radiolabel remaining in the plasma, whereas the steers not injected with Cu only had 40–50 % of the administered radiolabel (from tri-thiomolybdate) in the plasma (mainly in the TCA-insoluble fraction)(Reference Hynes, Woods and Poole14). Price et al. (Reference Price, Will and Paschaleris12) from their own and the work of others (discussed above) concluded that tetra-thiomolybdate was produced in the rumen, but remained absent from the liquid fraction and available for absorption until the solid fraction became saturated. The liquid fraction was the supernatant fraction of strained rumen fluid (through a single layer of muslin) after centrifugation at 2200 g, the solid fraction being the fine particulate material. The saturation of the solid fraction is probably related to the ruminally available Cu content of the solid fraction and this is at least partially shown by the Cu supplementation in the work of Hynes et al. (Reference Hynes, Woods and Poole14) discussed above. This is supported by work by Price & Chesters(Reference Price and Chesters16) that showed available Cu in the digesta to be mainly in the solid phase of the rumen fluid. It is further supported by the tetra-thiomolybdate having a greater affinity for Cu and therefore the tetra-thiomolybdates will react with available Cu before the tri- and subsequently di-thiomolybdates and therefore not be available for absorption whereas the less reactive species are. Price et al. (Reference Price, Will and Paschaleris12) showed the presence of Cu(I)tetra-thiomolybdate (CuS2MoS2Cu) in rumen digesta as well as Cu(I)tri-thiomolybdate and Cu(I)di-thiomolybdate. This Cu(I)tetra-thiomolybdate was also found to have completely exchangeable tetra-thiomolybdate given excess copper iodide.
Suttle & Field(Reference Suttle and Field17) also concluded that tetra-thiomolybdates caused effects of Cu and Mo antagonism, whereas the di-thiomolybdate was shown not to play an important role in the Cu and Mo antagonism.
What effects do absorbed thiomolybdates have in the animal?
Chidambaram et al. (Reference Chidambaram, Barnes and Frieden18) showed tetra-thiomolybdate to inhibit in vitro all of the following Cu-containing oxidase enzymes (value in parentheses is the concentration of tetra-thiomolybdate required for 50 % inhibition): caeruloplasmin (p-phenylenediamine 3·0 μm; o-dianisidine 2·7 μm); cytochrome oxidase (2·0 μm); superoxide dismutase (5·0 μm); ascorbate oxidase (1·0 μm); tyrosinase (dopachrome formation 3·0 μm, catecholase acivity 4·5 μm). These levels of inhibition are physiologically relevant. Phillippo et al. (Reference Phillippo, Humphries and Atkinson19) measured plasma Mo concentrations ranging from below the detection limit of 0·015 μg/ml (0·156 μm) for control and Fe-supplemented treatments up to 0·16 μg/ml (1·67 μm) for Mo-supplemented groups which would indicate that these levels could potentially be achieved in plasma, with the large caveat that it is not possible to show how much of the plasma Mo is in the form of thiomolybdates.
Chidambaram et al. (Reference Chidambaram, Barnes and Frieden18) also showed using atomic absorption that caeruloplasmin and tetra-thiomolybdate-treated caeruloplasmin both contained six coppers, i.e. tetra-thiomolybdate does not remove Cu from caeruloplasmin to inactivate it. The tetra-thiomolybdate was suggested to bind to the caeruloplasmin through the sulfide ( − S) groups, reducing the Cu (II) to Cu (I) (Fig. 3).
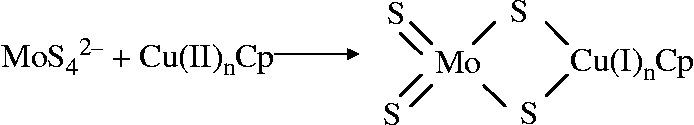
Fig. 3 Reaction for the binding of thiomolybdate to caeruloplasmin (Cp)(Reference Chidambaram, Barnes and Frieden18).
Chidambaram et al. (Reference Chidambaram, Barnes and Frieden18) also showed that, on a mole basis, ten tetra-thiomolybdate molecules bind to each caeruloplasmin, which suggests that some non-specific binding of tetra-thiomolybdate to caeruloplasmin must also occur; this had been previously shown to occur with non-specific binding of tetra-thiomolybdate to albumin. This reaction was reported to be irreversible. However, both Hynes et al. (Reference Hynes, Woods and Poole14) and Lannon & Mason(Reference Lannon and Mason20) reported reversibility of tri- and tetra-thiomolybdate effects both in vivo and in vitro. In vitro, Lannon & Mason(Reference Lannon and Mason20) showed the reaction to reverse after gel filtration of the plasma, whilst in vivo the reaction began to reverse on cessation of the tetra-thiomolybdate infusion.
Lannon & Mason(Reference Lannon and Mason20) showed that infusion of tetra- and tri-thiomolybdates both reduced the caeruloplasmin p-phenylenediamine oxidase activity and TCA solubility of Cu, with the tetra form more potent than the tri form. They also found an increase in Cu associated with albumin. The reaction was apparently reversible, as full p-phenylenediamine oxidase activity of caeruloplasmin returned in vitro after gel filtration of the plasma, and in vivo as activity began to return upon cessation of thiomolybdate infusion. It was also noted that during infusion the total Cu content of the blood increased (24 % over 24 h); however, there was no reduction in caeruloplasmin Cu associated with the increased Cu, which is in agreement with Chidambaram et al. (Reference Chidambaram, Barnes and Frieden18) showing that Cu is not removed from the caeruloplasmin to reduce the enzymic activity. The increase in plasma Cu is something also shown by Kendall et al. (Reference Kendall, Illingworth, Campbell, Bonham, Duffy, McAnena and Strain21), who found the total Cu concentration of plasma to be increased by a continuous infusion of 1 mg tetra-thiomolybdate per h in sheep. By 1-week post-cessation of infusion, thiomolybdate and control infused sheep had similar concentrations of plasma Cu. The TCA-soluble plasma Cu concentration remained consistent with the tetra-thiomolybdate infusion having a significant TCA-insoluble fraction. The caeruloplasmin p-phenylenediamine oxidase activity was also decreased by the tetra-thiomolybdate infusion, but at the 1-week post-infusion sample full activity was regained. It was only the measure of whole blood superoxide dismutase activity which remained depressed at 1 week after cessation of the 5 d tetra-thiomolybdate infusion.
Kumaratilake & Howell(Reference Kumaratilake and Howell22) found in Cu-loaded sheep (fed 2 % solution of copper sulfate at 10 ml/kg body weight for 5 d per week until a rise in alkaline phosphatase was observed) (range 55–149 d) that the administration of tetra-thiomolybdate (50 μg intravenously twice per week) for 11 weeks significantly reduced liver Cu concentrations from 813·76 (sd 74·77) to 305·78 (sd 48·25) mg/kg wet weight (ww); Cu concentrations also decreased in the non-thiomolybdate-treated controls but only to 594·57 (sd 104·29) mg/kg ww. Also there were significantly increased liver Mo concentrations (0·93 to 87·25 (sd 16) mg/kg ww) in the Cu-loaded animals.
Haywood et al. (Reference Haywood, Dincer and Jasani23) found a pituitary endocrinopathy in sheep associated with therapeutic administrations of thiomolybdates for the treatment of Cu toxicity. Williams et al. (Reference Williams24, Reference Williams, Haywood, Wilkinson, Bonham, Duffy, McAnena and Strain25) have shown similar effects in sheep feed Mo and S.
The role of iron: how does it react in the rumen?
Phillippo et al. (Reference Phillippo, Humphries and Atkinson19, Reference Phillippo, Humphries, Bremner, Mills, Bremner and Chesters26) showed Fe and S supplementation to reduce the blood plasma Cu content of cattle to hypocupraemic status (1·89–2·67 μm and 3·15–3·78 μm compared with control group minimums of 10·54 and 15·73 μm for two experiments, respectively; the internationally recognised threshold for Cu deficiency is 9.4 μm(Reference Radostits, Gay and Hinchcliff27)) without inducing the symptoms of ‘clinical Cu deficiency’ (reduced weight gain, decreased food intake, reduced efficiency of food conversion, alteration in hair texture, delayed puberty, reduced conception rate and inhibition of oestrus). Fe and Mo supplementation both reduced liver Cu and plasma Cu concentrations. Bremner et al. (Reference Bremner, Humphries and Phillippo28) showed that Fe supplementation (4.5, 9 and 13·5 mmol Fe/kg DM) had no effect on Cu status in preruminant calves, but decreased plasma and liver Cu when fed post-wearing although this did not induce a ‘clinical’ Cu deficiency until Mo was also fed. The effects of Fe and Mo were additive at the relatively low levels administered; previous trials with high supplement rates did not indicate an additive effect.
Campbell et al. (Reference Campbell, Coup and Bishop29) showed Fe supplementation (30 mg/kg live weight) to reduce liver Cu from 179 mg/kg DM down to 7 mg/kg DM, with plasma Cu concentrations starting to reduce after depletion of liver Cu to 40 mg/kg DM. The Cu-dependent enzymes amine oxidase and caeruloplasmin were measured in this trial only after blood Cu had already been significantly reduced and were lower for the Fe-dosed group compared with the control group. These animals had a basal diet containing 1·5 mg Mo/kg DM and 10·7 mg Cu/kg DM.
No effect of high Fe supplementation (1 g/kg diet) was noted on serum, liver or spleen Cu concentration by Rosa et al. (Reference Rosa, Ammerm and Henry30); however, these sheep were being fed a low-S diet.
Standish & Ammerman(Reference Standish and Ammerman31) showed iron sulfate (1·6 g/kg Fe, 0·28 % sulfate) to significantly reduce the blood Cu concentration when compared with iron citrate (1·6 g/kg Fe) and no supplement. However, blood Cu concentrations when fed sodium sulfate (0·28 % sulfate) were reduced but not significantly, and not to the degree that the iron sulfate reduced the blood Cu concentration.
In the rumen the Fe can react with sulfide and Cu either to produce an Fe–Cu–S complex which is not absorbed by the animal; an alternative hypothesis is that the Fe and sulfide combine to form an iron sulfide, and the Cu then exchanges with the Fe to form a copper sulfide (comment by C. F. Mills in Suttle & Peter(Reference Suttle, Peter, Mills, Bremner and Chesters32)). Both will therefore reduce the amount of Cu available within the rumen and will hence reduce the Cu available to participate in the Mo and S (thiomolybdate) and Cu interactions. However, there is little direct evidence in the literature to support either hypothesis, although what is clear is that there is an Fe–Cu–S interaction and it does decrease Cu availability.
Sources of copper, molybdenum, sulfur and iron
The complex interaction between these elements is explained above, but it is important to remember how these elements reach the rumen and in what form. The major routes of entry are in the diet and in the drinking water. The diet includes the elemental composition of the plant material consumed, dietary inclusion and supplements containing minerals (for example, mineral licks) and soil consumption, both as feed contamination and direct consumption. Soil can account for up to 25 % of DM intake in certain situations; for example, in sheep grazing pasture with short grass either in very wet conditions (poaching) or with very dry and dusty conditions, where the annual soil consumption can exceed the live weight of the animal(Reference Healy, Hoekstra, Suttie, Ganther and Mertz33). Other sources confirm this, with over-wintered grazing cattle ingesting between 140 and 1400 g soil per d(Reference Thornton, Hoekstra, Suttie, Ganther and Mertz34). Drinking water can also be an important elemental entry route, whether it is mains water, spring water, stream water or from a borehole. Borehole water, especially, tends to be individual to the farm and can have a high sulfide content which as outlined above makes the S much more potent in the thiomolybdate formation reactions. The Fe in borehole water also often tends to be in the highly reactive ferrous form.
Water-logging of soil in addition to increasing poaching and increasing soil consumption will also increase the uptake of Fe to plant material (four- to five-fold in young green shoots), with the Fe in the highly bioactive ferrous form(Reference Campbell, Coup and Bishop29). When soil (contamination) is ensiled with forages the fermentation process appears to increase the Fe bioactivity(Reference Hansen and Spears35). The Mo uptake by plants, especially legumes, is also increased when grown in wet soils(Reference Kubota36). In general, Mo content is higher in vegetable protein supplements (for example, soya) and legumes (for example, clover)(Reference Mills, Davis, Mertz and Underwood37). Mo uptake is also increased with increasing pH of the soil (liming pasture increases Mo uptake) and generally increases throughout the growing season(Reference Mills, Davis, Mertz and Underwood37). S tends to be related to protein concentration.
There are also differences found between continuous and discontinuous feeding on sulfide production(Reference Suttle, Peter, Mills, Bremner and Chesters32); this will also affect rumen pH. Therefore, a continuous feeding system (for example, total mixed ration; TMR) will give a more consistent but moderately higher rumen pH with a stable sulfide content whereas a single or twice-daily feed will give a rapid decrease in rumen pH and peak production of sulfide, both of which will revert over time before the next meal.
Summary
Within the present review we have been able to provide evidence that: all classes of thiomolybdates are formed in the rumen; in the absence of available Cu all thiomolybdates can be absorbed into the animal rapidly though the rumen wall or more sedately via the small intestine; thiomolybdates can bind to Cu in biological compounds and are able to cause problems; effects of thiomolybdate are reversible in vivo and in vitro on cessation of thiomolybdate challenge; the tetra-thiomolybdate form is the most potent Cu binder with decreased potency with decreased S in the compound. Fe will exacerbate a thiomolybdate problem but will not directly cause it.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
N. K. was the major contributor. L. G. had some small but significant input.
There are no conflicts of interest.





