Introduction
Chronic periods of reduced physical activity occur throughout the life, following injuries or trauma, after prolonged immobilisation (casting or extended bed rest), or as a natural result of ageing. The primary effect of muscle disuse in such situations is the progressive loss of skeletal muscle, even in the absence of a diagnosed disease(Reference Blottner, Salanova and Puttmann1–Reference Pavy-Le, Heer and Narici3). For example, ageing itself accelerates inactivity-induced atrophy(Reference English and Paddon-Jones4), hence contributing to the decline of muscle function and physical activity in older adults(Reference Gill, Allore and Guo5). Since skeletal muscle is the major reservoir of body proteins and amino acids that the organism could mobilise to cope with traumatic, infectious or nutritional stresses, sustained and uncontrolled muscle mass loss is then deleterious for maintaining optimal health status. After a period of immobilisation, the rehabilitation phase usually leads to a complete muscle mass recovery. Even though mechanisms involved during disuse-induced atrophy have been extensively studied, mechanisms involved in the recovery phase have not been thoroughly investigated. Exercise is known to be the best way to maintain and/or gain muscle mass even in elderly individuals(Reference Nair6–Reference Yarasheski, Pak-Loduca and Hasten8), but it is not always applicable in all situations. Thus, other approaches such as nutritional strategies are needed to limit muscle wasting and to improve muscle mass recovery in such situations. The purpose of the present review is to concentrate on mechanisms involved in muscle atrophy following disuse and during recovery and to discuss the efficiency of potential nutritional strategies to limit muscle mass loss during disuse and to improve muscle mass gain during recovery in such situations of immobilisation.
Protein turnover during muscle disuse
Due to a decrease in mechanical stimuli, muscle disuse induces an alteration in skeletal muscle protein turnover associated with a negative N balance(Reference Ferrando, Lane and Stuart9–Reference Stevenson, Giresi and Koncarevic12). A decreased synthesis and/or an increased breakdown of myofibrillar proteins (which represent about 85 % of the muscle fibre volume) explain muscle atrophy and the subsequent muscle dysfunction(Reference Boonyarom and Inui13, Reference Morris, Morris and Kennedy14). Although the direct measurement of muscle protein breakdown is not possible in vivo, surrogate markers such as proteolytic enzyme activities or mRNA have been used to describe myofibrillar proteolysis during immobilisation. Experiments with immobilisation conducted in human subjects and animals indicated that muscle disuse induces an early increase in protein breakdown, within the first 24 h(Reference Jones, Hill and Krasney15, Reference Tesch, von Walden and Gustafsson16). The extent of this increase differs considerably according to the model of immobilisation used, varying from 50 to 200 %(Reference Jones, Hill and Krasney15, Reference Glover, Yasuda and Tarnopolsky17–Reference Zdanowicz and Teichberg21). All the proteolytic systems, which target different components of muscle architecture, seem to be involved. The main proteolytic system activated during immobilisation is the ubiquitin–proteasome system (UPS). This is, somehow, not very surprising as the UPS is responsible for the breakdown of the major contractile proteins, i.e. actin and myosin(Reference Attaix, Ventadour and Codran22–Reference Polge, Heng and Jarzaguet24). In this pathway, substrates are tagged by covalent attachment of multiple ubiquitins and then recognised and degraded by the 26S proteasome. Both steps are activated during bed rest in humans(Reference Jones, Hill and Krasney15, Reference Glover, Yasuda and Tarnopolsky17) or immobilisation-induced atrophy in animals(Reference Ikemoto, Nikawa and Takeda25–Reference Vazeille, Slimani and Claustre29). Animal models of immobilisation also clearly demonstrated that the UPS is then rapidly normalised during the recovery period in adults(Reference Taillandier, Aurousseau and Combaret27, Reference Vazeille, Slimani and Claustre29). The same kinetics has been observed in aged animals immobilised by casting(Reference Magne, Savary-Auzeloux and Vazeille30, Reference Magne, Savary-Auzeloux and Migne31); however, in these experiments the UPS was activated to a lesser extent: the proteasome chymotrypsin-like activity increased only by 53 % in old rats, while a 138 % increase was reported in adult rats immobilised using the same model(Reference Vazeille, Codran and Claustre28). As immobilisation-induced muscle atrophy remained similar at both ages, with 21 and 23 % in old and adult rats, respectively, this indicates that additional proteolytic mechanisms leading to muscle loss during disuse may take place during ageing(Reference Pattison, Folk and Madsen32, Reference Pattison, Folk and Madsen33). Calpains are proteases involved in the early steps of disassembly of sarcomeric proteins(Reference Attaix, Ventadour and Codran22, Reference Hasselgren and Fischer34, Reference Huang and Forsberg35). The role of the calpain pathway in response to immobilisation was demonstrated in vivo and in vitro (Reference Stevenson, Giresi and Koncarevic12, Reference Taillandier, Aurousseau and Meynial-Denis26, Reference Goll, Thompson and Li36) and confirmed in mice by using inhibitors of calpain activity which prevented sarcomere structure disruption(Reference Salazar, Michele and Brooks37). In healthy volunteers submitted to limb immobilisation, an early (24 h) increase of mRNA coding for calpains-1 and -2 has been observed(Reference Jones, Hill and Krasney15). Growing evidence also suggests a central role of the lysosomal pathway (i.e. autophagy processes) in immobilisation-induced atrophy, despite its low contribution to overall protein breakdown and weak participation in the degradation of myofibrillar proteins(Reference Attaix, Ventadour and Codran22). Key molecules of this pathway (i.e. Beclin 1 or LC3) are nevertheless over-expressed in response to muscle disuse within the first 14 d of immobilisation(Reference Liang, Jackson and Seaman38) and cathepsin mRNA, and activities which are protease-specific to this pathway are also increased(Reference Taillandier, Aurousseau and Meynial-Denis26, Reference Andrianjafiniony, Dupre-Aucouturier and Letexier39–Reference Zhao, Brault and Schild41). Despite autophagy-lysosome substrates in skeletal muscle being still poorly identified, this pathway could nevertheless play a crucial role during disuse as its activation is associated with the activation of the forkhead O transcription factors (FoxO) which are known to activate the muscle RING-finger protein-1 (MuRF1) and the specific muscle ubiquitin ligase muscle atrophy F-box (MAFbx) atrogenins (atrogens) involved in the activation of UPS proteolysis(Reference Zhao, Brault and Schild41).
Protein synthesis is the most dynamic variable in the protein balance and it changes several-fold throughout the day, as demonstrated with direct measurement(Reference Phillips, Glover and Rennie10). However, when studied in the fasted state, contradictory results have been reported with either a reduced basal-fasted rate of muscle protein synthesis(Reference Biolo, Ciocchi and Lebenstedt42, Reference Gibson, Halliday and Morrison43), or an unchanged rate of protein synthesis(Reference Lovejoy, Smith and Zachwieja44–Reference Stuart, Shangraw and Peters47). When muscle protein synthesis was decreased, this change occurred early during the immobilisation period ( < 10 d) and then protein synthesis stabilised, i.e. remained decreased at the same level for the next 21 d of immobilisation(Reference de Boer, Selby and Atherton48). Conversely, data regarding the postprandial state agreed that immobilised skeletal muscle exhibits a decrease in responsiveness of muscle protein synthesis to amino acids across a wide range of amino acid concentrations. Indeed, provision of low and very high concentrations of amino acids (43 and 261 mg/kg per h, respectively) failed to restore muscle protein synthesis to that seen in the non-immobilised muscle(Reference Glover, Phillips and Oates49). Immobilisation may thus induce a real resistance of skeletal muscle protein synthesis to the stimulatory effect of food intake(Reference Phillips, Glover and Rennie10, Reference Glover, Phillips and Oates49), a condition defined as ‘anabolic resistance’(Reference Glover, Phillips and Oates49). Under conditions of anabolic resistance, the normal anabolic response to amino acids is blunted and the subsequent postprandial gain in muscle proteins may be less important, resulting in muscle atrophy(Reference Rennie, Wackerhage and Spangenburg50). This explains why a normal diet (i.e. which in theory covers the recommended daily amino acid requirements) is a diet not adapted any more to overcome anabolic resistance(Reference Dardevet, Remond and Peyron51) and why animals or individuals fed such a diet present this decrease in postprandial protein synthesis when experiencing prolonged immobilisation. Recently, this anabolic resistance has also been demonstrated in aged animals immobilised by casting(Reference Magne, Savary-Auzeloux and Migne31), which could exacerbate the age-related ‘normal’ blunted response of muscle anabolism to food intake (see the section on Ageing in the present review). The rate of protein synthesis is dependent on the stimulation and the efficiency of the anabolic phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway. Immobilisation induces a decrease in Akt protein content(Reference Bodine, Stitt and Gonzalez52), but its activity is either decreased(Reference O'Keefe, Perez and Sloniger53, Reference Sugiura, Abe and Nagano54) or unchanged(Reference Hunter, Stevenson and Koncarevic55, Reference Morris, Spangenburg and Booth56), which, in both cases, leads to an imbalance of protein synthesis as protein breakdown is increased. Muscles from animals with immobilised hindlimbs exhibit a decrease in S6 kinase 1 (S6K1) protein content(Reference You, Park and Song57), and a decrease in mRNA translation, a critical step of protein synthesis(Reference Booth and Kirby58) dependent on the activation of mTOR. However, data from recent disuse investigations in human subjects have failed to demonstrate a decrease in activation of key proteins in the PI3K/Akt/mTOR pathway during both the postabsorptive and the postprandial states, suggesting that the decrease in protein synthesis may be regulated by downstream translation, initiation and elongation factors independent of PI3K/Akt/mTOR signalling(Reference de Boer, Selby and Atherton48, Reference Glover, Phillips and Oates49). Confirming this hypothesis, data have shown that 10 or 21 d bed rest in humans did not affect the phosphorylation state of S6K1 and 4E-BP1 proteins in the postabsorptive state(Reference de Boer, Selby and Atherton48). This demonstrates the complexity of the regulation involved, which may include: (1) species differences in protein metabolism; (2) altered kinetics of protein synthesis during immobilisation; and (3) the nutritional state (postabsorptive or postprandial) during which the measure is performed.
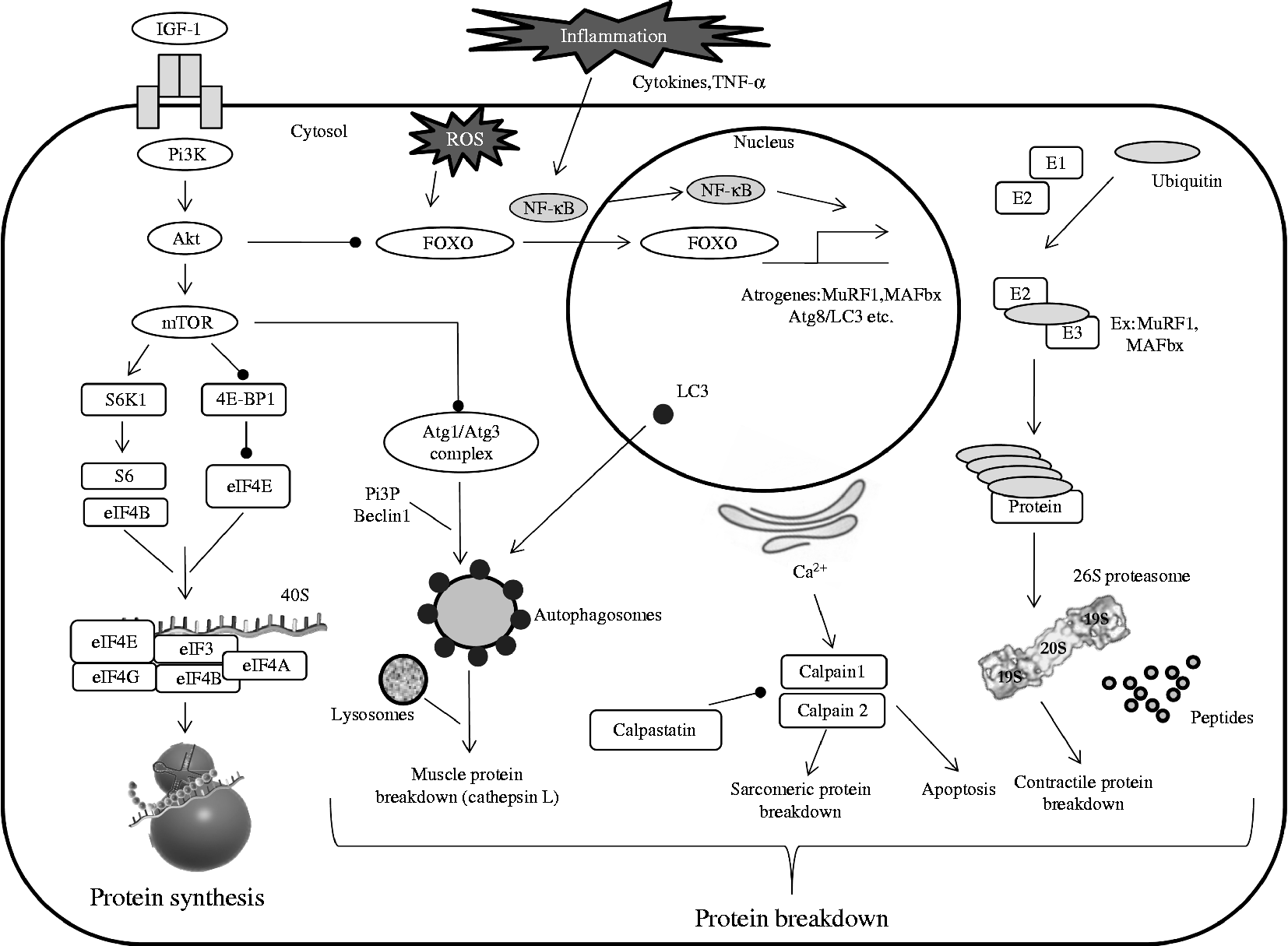
Two possible links could exist between skeletal muscle protein breakdown and protein synthesis (Fig. 1). The first one is the specific MAFbx (a main component of the UPS) which is known to orientate eukaryotic initiation factor (eIF)-3 subunit f (eIF3-f) to degradation by the proteasome(Reference Lagirand-Cantaloube, Offner and Csibi59). Thus, part of the decreased protein synthesis observed during immobilisation could indirectly result from the activation of the protein breakdown pathway(s). The second ones are the FoxO transcription factors that regulate the specific muscle ubiquitin ligases MAFbx and MuRF1 directly or indirectly(Reference Sandri, Sandri and Gilbert60, Reference Sandri61). Over-expression of FoxO1 and FoxO3 increases the expression of MuRF1 and MAFbx and then reduces muscle mass during immobilisation(Reference Kandarian and Jackman62, Reference Romanello, Guadagnin and Gomes63). Similarly, immobilisation for 1 week increased FoxO1 activity and MuRF1 and MAFbx levels(Reference Bae, Cha and Ju64). Several experiments have demonstrated a connection of the reduced activation of the PI3K/Akt/mTOR pathway and the decrease in skeletal muscle protein synthesis with the dephosphorylation of FoxO factors and the increased expression of proteolytic genes(Reference Bodine, Stitt and Gonzalez52, Reference Kandarian and Jackman62, Reference Guttridge65–Reference Stitt, Drujan and Clarke67). The results from immobilisation experiments are consistent with these data, as Akt kinase is known to phosphorylate the FoxO factors, which are then unable to translocate into the nucleus and to induce the transcription of proteolytic genes. Interestingly, while activities of Akt and mTOR were both reduced after 6 h or 1 week of immobilisation, the expression level of both MuRF1 and MAFbx ligases was reduced only after 1 week of immobilisation, suggesting that protein synthesis is affected earlier than protein degradation(Reference Bae, Cha and Ju64) and is the primary driver of immobilisation-induced atrophy.

Fig. 1 Simplified pathways regulating muscle protein metabolism. Maintenance of muscle mass depends on muscle protein synthesis which is normally balanced with muscle protein breakdown. Muscle proteolysis involves three main pathways (ubiquitin-dependent system, lysosomal and calpain-dependent pathways). Regulation involves complex processes between systems. PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; Akt, protein kinase B; FOXO, forkhead O transcription factor; MuRF1, muscle RING-finger protein-1; MAFbx, muscle ubiquitin ligase muscle atrophy F-box; Atg, autophagy-related; LC3, light chain 3; E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme; E3, ubiquitin ligase; mTOR, mammalian target of rapamycin; S6K1, S6 kinase 1; 4E-BP1, factor 4E binding protein 1; eIF, eukaryotic initiation factor; PI3P, phosphatidylinositol 3-phosphate.
Cellular turnover during muscle disuse
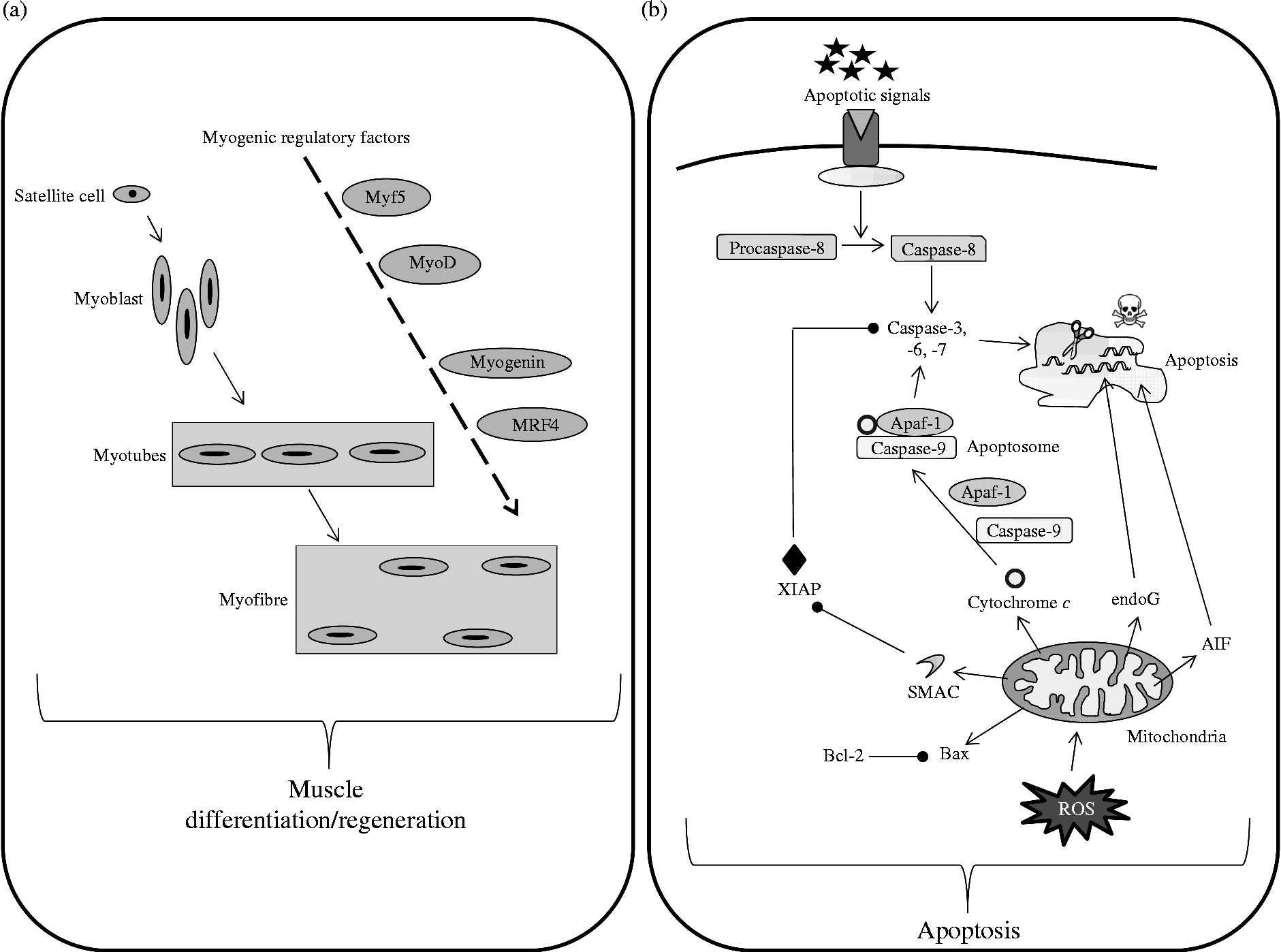
Muscle atrophy that occurs in immobilised muscles is also associated with a loss of functional muscle cells(Reference Allen, Yasui and Tanaka68–Reference Smith, Maxwell and Martyn70). This observation has been linked to an increase in apoptotic processes in various animal models of immobilisation(Reference Vazeille, Codran and Claustre28, Reference Leeuwenburgh, Gurley and Strotman69–Reference Schmalbruch and Lewis72) (Fig. 2). This increased apoptosis seems to be more detectable in slow-type fibres(Reference Allen, Yasui and Tanaka68, Reference Leeuwenburgh, Gurley and Strotman69, Reference Allen, Linderman and Roy73), which could partly explain why this fibre type is more likely to be altered by immobilisation. Among the multiple pathways leading to cellular apoptosis, mitochondria-mediated apoptosis is deeply involved in the atrophy of disused muscle(Reference Vazeille, Codran and Claustre28, Reference Vazeille, Slimani and Claustre29, Reference Marzetti, Hwang and Lees74, Reference Powers, Smuder and Judge75) and to the same extent whatever the age(Reference Vazeille, Codran and Claustre28–Reference Magne, Savary-Auzeloux and Vazeille30). To our knowledge no data on apoptosis in response to muscle disuse by itself and without any pathology are available in humans.

Fig. 2 Simplified pathways regulating muscle cellular balance. Cell number in the skeletal muscle is regulated by cell differentiation/regeneration (involving myogenic regulatory factors acting in cascades) (a) and cell apoptosis (b). Cell apoptosis has been simplified and normally involves three different pathways, i.e. external, internal caspase-dependent and internal caspase-independent. Myf5, myogenic factor 5; MyoD, myogenic differentiation antigen; MRF4, myogenic regulatory factor 4; Apaf-1, apoptotic protease activating factor 1; endoG, endonuclease G; XIAP, X-linked inhibitor of apoptosis protein; AIF, apoptosis inducing factor; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; ROS, reactive oxygen species.
Muscle size is dependent on the number and volume of muscle fibres, which is directly related to satellite cells. These quiescent and undifferentiated cells are activated from the quiescent state upon appropriate stimulatory signals and undergo active proliferation and myogenic differentiation. Mechanical unloading, as observed during immobilisation, has been shown to reduce the number of satellite cells, probably due to impaired function and/or activity of these satellite cells(Reference Darr and Schultz76–Reference Mozdziak, Truong and Macius78). This could be explained by a reduction in the amount and/or activity of the myogenic regulatory factors responsible for the activation of satellite cells and this has been shown in casted animals with myf5(Reference Vazeille, Codran and Claustre28, Reference Magne, Savary-Auzeloux and Vazeille30) and in suspended animals with myoD and myogenin(Reference Shefer, Carmeli and Rauner79, Reference Zhang, Yeung and Liu80) (Fig. 2). As a relationship between muscle fibre size and myonuclear number exists, the myonuclear domain has been defined as ‘cytoplasmic volume to nucleus ratio’ and refers to a capacity of the cell to supply transcriptional demands(Reference Van der Meer, Jaspers and Degens81). This myonuclear domain is normally constant, but decreased during immobilisation particularly in slow-type fibres, hence probably contributing to the muscle atrophy(Reference Allen, Monke and Talmadge82–Reference Tseng, Kasper and Edgerton84).
Metabolic abnormalities associated with disuse
Physical inactivity is associated with a disruption of glucose homeostasis. Thus, insulin resistance appears during prolonged periods of immobilisation(Reference Reynet and Kahn85, Reference Vaag86). However, the specific molecular mechanisms underlying this association remain unclear(Reference Handschin and Spiegelman87). This insulin resistance has been found even in young healthy men with and without a known predisposition to diabetes(Reference Alibegovic, Hojbjerre and Sonne88–Reference Alibegovic, Sonne and Hojbjerre90), confirming the role of immobilisation by itself. This decreased insulin sensitivity appears early during the immobilisation period (after only 3–5 d)(Reference Dolkas and Greenleaf91–Reference Tabata, Suzuki and Fukunaga96). Since insulin is known to stimulate protein synthesis in gastrocnemius muscles(Reference Kimball, Jurasinski and Lawrence97), this decreased insulin sensitivity could contribute to the anabolic resistance observed. Moreover, muscle disuse induces a metabolic shift towards utilisation of glucose away from fat as evidenced by increased gene expression of proteins involved in glycolysis and decreased expression of proteins involved in β-oxidation in hindlimb atrophied soleus muscle(Reference Stein, Schluter and Galante98). These metabolic changes are important because they affect performance and may affect recovery processes.
Furthermore, growing evidence suggests that skeletal muscle atrophy is positively correlated with oxidative stress in various situations of muscle disuse(Reference Koesterer, Dodd and Powers99–Reference Lawler, Song and Demaree106) despite there being no clear evidence to determine if it is a primary cause or a latter consequence of disuse. This oxidative stress is characterised by an increase in reactive oxygen species in skeletal muscle of both animals(Reference Candelario-Jalil, de Oliveira and Graf107, Reference Murcia and Martinez-Tome108) and humans(Reference Lawler, Song and Demaree106, Reference Agostini, Dalla and Rittweger109). It has been postulated that this oxidative stress may play an important role in the process of muscle atrophy during disuse(Reference Powers, Smuder and Judge75). Even if its origin remains to be clearly determined(Reference Powers, Kavazis and McClung110), one of the possible mechanisms could be a decreased production of endogenous antioxidant defences in response to immobilisation(Reference Stevenson, Giresi and Koncarevic12, Reference Crowe, McArdle and McArdle111–Reference Stein113), due to the decrease in protein synthesis associated with muscle atrophy(Reference Stevenson, Giresi and Koncarevic12, Reference Sacheck, Hyatt and Raffaello114). In rodents, immobilised muscles exhibit either a decrease(Reference Kondo, Miura and Nakagaki101, Reference Ikemoto, Okamura and Kano115) or an increase(Reference Magne, Savary-Auzeloux and Vazeille30) in muscle glutathione concentration. The activity of key enzymes in glutathione metabolism such as glutathione reductase and glutathione peroxidase are increased(Reference Sen, Marin and Kretzschmar116), unaffected or down-regulated(Reference Tauler, Aguilo and Gimeno117). When reactive oxygen species increase in skeletal muscle, DNA fragmentation, lipid peroxidation and protein oxidation can result, which can lead to apoptosis, depending on the severity of the oxidative stress(Reference Leeuwenburgh, Gurley and Strotman69, Reference Cai, Kang and Liu118, Reference Nagano, Suzaki and Nagano119). As an example, a large increase in carbonylated protein content was demonstrated in human subjects following 35 d of bed rest(Reference Agostini, Dalla and Rittweger109). When carbonylated, proteins are preferentially degraded by the 20S proteasome without ubiquitination(Reference Grune, Merker and Sandig120) and there is evidence that these damaged proteins are more efficiently and rapidly scavenged by proteolytic degradation than their non-oxidised forms to avoid accumulation of damaged functional proteins(Reference Grune, Merker and Sandig120–Reference Dukan, Farewell and Ballesteros122). Thus, an increase in protein carbonylation could lead to an increase in proteolysis, hence contributing to muscle atrophy. Moreover, oxidative stress is an important activator of key proteases (for example, calpain and caspase-3) in skeletal muscle(Reference Smuder, Kavazis and Min123, Reference Whidden, Smuder and Wu124) whose activities have been demonstrated to be increased in immobilised muscle(Reference Siu, Pistilli and Alway125). Finally, excess production of reactive oxygen species can also up-regulate NF-κB activity, which in turn may also enhance protein degradation by the UPS(Reference Bar-Shai, Carmeli and Ljubuncic126–Reference Powers and Lennon128).
Finally, immobilisation-induced atrophy is associated with local inflammation, as demonstrated in human subjects and animals(Reference Magne, Savary-Auzeloux and Vazeille30, Reference Andrianjafiniony, Dupre-Aucouturier and Letexier39). This inflammatory response parallels tissue infiltration by macrophages(Reference Appell129–Reference Bigard, Merino and Lienhard131). These inflammatory cells are able to produce the cytokine TNF-α which could later activate the inflammatory NF-κB pathway(Reference Powers, Smuder and Judge75, Reference Powers, Kavazis and McClung110). Up-regulation of other pro-inflammatory cytokines (for example, IL-1β and IL-6) has also been observed in response to muscle immobilisation(Reference Andrianjafiniony, Dupre-Aucouturier and Letexier39). As these cytokines are able to activate apoptosis and proteolysis in muscle(Reference Cai, Frantz and Tawa132, Reference Pistilli, Jackson and Alway133), this immobilisation-induced inflammation may participate directly to the generation of muscle atrophy. Indeed, it has been well demonstrated under other atrophying conditions that inflammation is the main negative regulator of skeletal muscle protein synthesis(Reference Lang, Frost and Nairn134–Reference Rieu, Magne and Savary-Auzeloux136).
Protein and cellular turnover during the muscle recovery phase
After a catabolic state, muscle mass recovery is a key factor in maintenance of the health and autonomy of individuals. Two main processes are necessary to ensure an efficient recovery, namely a gain in muscle fibre number and muscle protein accretion. The appearance of new muscle fibres has been observed in human subjects as soon as 4 d after recovery and muscle mass loss has been demonstrated to be fully recovered after 14–40 d in animals depending on the model studied(Reference Vazeille, Codran and Claustre28, Reference Sugiura, Abe and Nagano54, Reference Mitchell and Pavlath137, Reference Oishi, Ogata and Yamamoto138). As mentioned previously, muscle regeneration and subsequent muscle fibre gain depend on the number and activity of satellite cells, and on their upstream regulators, i.e. the myogenic regulatory factors. Subsequent to 2 weeks of hindlimb unloading, a study(Reference Andrianjafiniony, Dupre-Aucouturier and Letexier39) explored inflammatory cytokines, apoptotic, or proteolytic pathways during the early (1 and 5 d) and later (14 d) stages of the regrowth process. It demonstrated that at early stages, muscle repair is mediated via the decrease in mitochondrial-driven apoptosis and proteolysis. Despite full muscle mass recovery, inflammation pathways were still activated until later stages of muscle remodelling(Reference Andrianjafiniony, Dupre-Aucouturier and Letexier39). These results and others(Reference Lawler, Song and Demaree106, Reference Childs, Spangenburg and Vyas139, Reference Washington, White and Davis140) demonstrated that inflammatory processes could be indispensable for an efficient remodelling and recovery of skeletal muscle, as inflammatory cytokines are needed to activate proliferation of muscle cells. The rapid recovery of muscle proteolysis and apoptosis has also been observed in several other studies(Reference Vazeille, Codran and Claustre28, Reference Vazeille, Slimani and Claustre29, Reference Allen, Sartorius and Sycuro141, Reference Fernando, Kelly and Balazsi142).
To generate the positive N balance required for protein accretion during recovery, a further increased protein synthesis, decreased proteolysis, or simultaneous changes in both processes is required. Only a few hours are needed for normalisation of the immobilisation-induced deregulated genes involved in protein turnover(Reference Sartorelli and Fulco143), hence improving whole-body protein synthesis and N retention(Reference Stein, Schluter and Galante98). Muscle protein breakdown is normalised early during the recovery period in adults(Reference Taillandier, Aurousseau and Combaret27, Reference Vazeille, Codran and Claustre28). Similarly, in adults, muscle protein synthesis is increased during the recovery period following immobilisation(Reference Booth144) via an increase of the Akt/mTOR pathway and downstream signalling(Reference Bodine, Stitt and Gonzalez52, Reference Sugiura, Abe and Nagano54, Reference Hornberger, Hunter and Kandarian145, Reference Reynolds, Bodine and Lawrence146) whereas it is only normalised in aged animals(Reference Magne, Savary-Auzeloux and Migne31). These results were confirmed in a study conducted in mice showing that mice heterozygous for mTOR with about 50 % reduction in total mTOR protein in skeletal muscle and other tissues failed to fully replete muscle mass during recovery following hindlimb immobilisation, whereas wild-type mice fully recovered(Reference Lang, Kazi and Hong-Brown147).
Nutrition: a key factor for preserving muscle from atrophy and improving recovery?
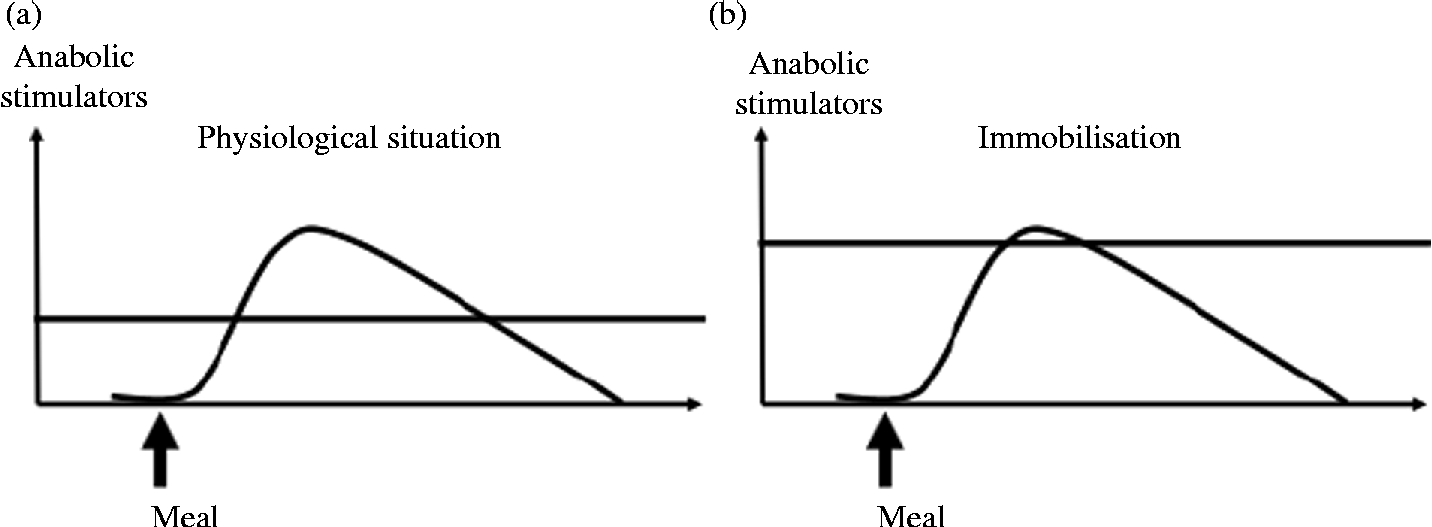
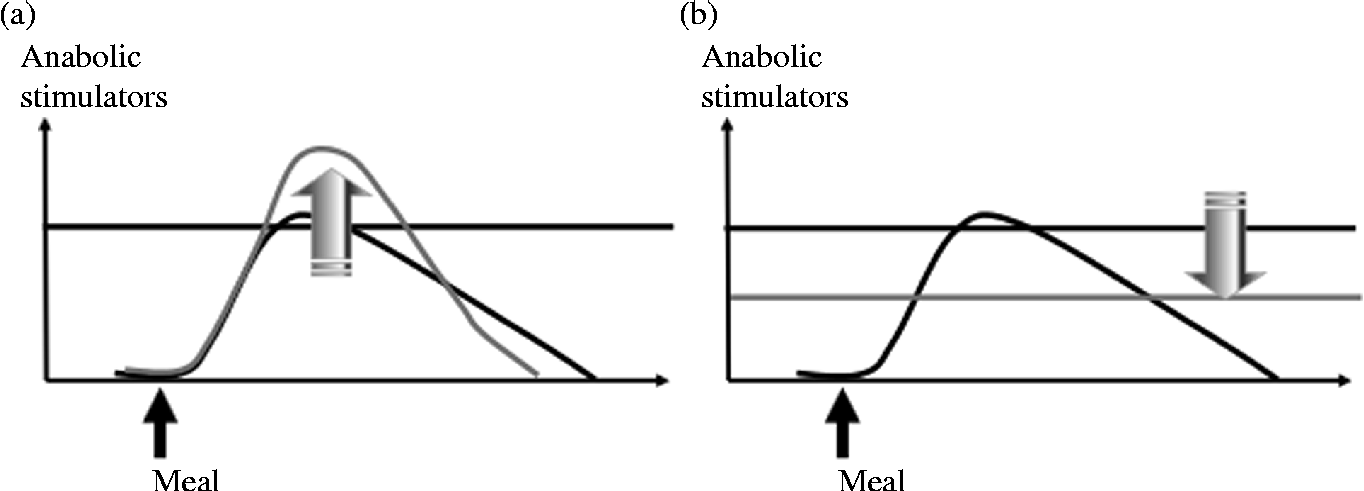
The maintenance of muscle mass during prolonged periods of immobilisation and the improvement of muscle mass gain during recovery are partly dependent on muscle protein turnover. Indeed, the anabolic resistance induced by immobilisation could represent the primary driver of muscle protein loss and subsequent atrophy. This phenomenon corresponds to an increased threshold of anabolism, i.e. a most important and more sustained amount of anabolic stimulators are required to obtain a stimulation of protein synthesis similar to the one before immobilisation (Fig. 3, adapted from Dardevet et al. (Reference Dardevet, Remond and Peyron51)). Nutritional strategies could be used during immobilisation and recovery to overcome this anabolic resistance. Two approaches are possible. The first one consists of exceeding this increased threshold (i.e. providing more anabolic factors, i.e. nutrients to achieve stimulation of protein synthesis), and the second one consists of decreasing the threshold (i.e. to restore muscle sensitivity to the stimulatory effect of normal food intake) (Fig. 4, adapted from Dardevet et al. (Reference Dardevet, Remond and Peyron51)).

Fig. 3 Anabolism threshold under normal (a) and bed rest (b) conditions. Muscle mass is regulated by the anabolism threshold which determines the efficiency of meal intake on muscle anabolism. Under bed rest conditions, anabolic resistance appears, i.e. the muscle anabolism threshold is increased, thus leading to poor efficiency of meal intake on muscle protein accretion.

Fig. 4 Nutritional strategies to overcome immobilisation-induced anabolic resistance. Two nutritional strategies could be proposed to overcome anabolic resistance during immobilisation: to increase the effect of anabolic factors (a) and/or to decrease/restore the anabolism threshold (b).
Nutritional intervention to exceed the increased anabolic threshold during immobilisation
Amino acid/protein supplementation
Protein and constitutive amino acids are known to be robust regulators of protein metabolism(Reference Anthony, Anthony and Layman148–Reference Koopman, Wagenmakers and Manders150). Therefore, the use of dietary protein and/or amino acid supplementation could be an efficient countermeasure to increase muscle anabolism during prolonged immobilisation and so to limit muscle atrophy.
As a normal diet is unable to maintain normal and sustained protein synthesis because of the increased threshold of anabolism, it is reasonable to think that increasing normal protein intake in bedridden patients might limit immobilisation-induced atrophy. Stuart et al. (Reference Stuart, Shangraw and Peters47) investigated the effects of increasing dietary protein intake isoenergetically from minimum requirements of 0·6–1·0 g/kg per d on whole-body protein synthesis during a 7 d bed rest study. They demonstrated that the decrease in protein synthesis induced by bed rest was prevented by the higher dietary protein intake and that N balance was improved. However, and unfortunately, muscle mass was not evaluated in this trial(Reference Stuart, Shangraw and Peters47). The protein source appeared to be a main determinant of the efficiency of nutritional interventions. Thus, casein has been referred to as a ‘slow’ protein because of the modest but prolonged increase in plasma amino acid concentrations following its ingestion. By contrast, whey proteins are defined as ‘fast’ proteins because they are rapidly digested and lead to a large, albeit temporary, rise in plasma amino acid levels(Reference Boirie, Dangin and Gachon151). Data indicate that the peak in plasma amino acids appeared later (150 min v. 75 min) and remained elevated longer after soya protein ingestion than whey protein ingestion(Reference Bos, Metges and Gaudichon152, Reference Farnfield, Trenerry and Carey153); this indicates that soya protein is more of an ‘intermediate’ protein in terms of digestion rate, based on plasma peak amino acid concentrations. In animals, dietary supplementation with 20 % soya protein hydrolysates reduced proteolytic pathways and was further able to prevent immobilisation-induced atrophy when compared with a 20 % casein control diet(Reference Tada and Yokogoshi154). As casein is the standard protein source used in animal diets, these results confirm that a normal diet is not suitable to limit muscle mass loss under conditions of disuse.
Essential amino acid (EAA) supplementations have also been proposed as a possible countermeasure in humans. Two studies of a 28 d bed rest trial did not show any benefit with such supplementations on either muscle mass or strength(Reference Brooks, Cloutier and Cadena155, Reference Brooks, Cadena and Vannier156). Another study tested EAA and carbohydrate supplementation during a 28 d bed rest trial in healthy subjects. Volunteers received a mix of 15 g EAA and 30 g sucrose (added for palatability) three times per d during the whole bed rest period. EAA supplementation increased protein synthesis throughout the study and maintenance of lean body mass was observed(Reference Paddon-Jones, Sheffield-Moore and Urban157). However, it is noticeable in this study that protein supplementation was associated with an increased energy intake and thus a synergic effect cannot be excluded(Reference Paddon-Jones, Sheffield-Moore and Urban157). Similar results with the same limitation (i.e. an increased energy intake) have been obtained by others in healthy males(Reference Fitts, Romatowski and Peters158). In this study, subjects consumed 16·5 g EAA and 30 g sucrose three times per d(Reference Fitts, Romatowski and Peters158). Altogether, these results suggest that EAA supplementation alone is unable to sufficiently stimulate muscle protein synthesis to counteract the deleterious effect of immobilisation.
Branched-chain amino acids (BCAA) and particularly leucine play an important role in the stimulation of postprandial muscle protein synthesis(Reference Anthony, Anthony and Layman148–Reference Koopman, Wagenmakers and Manders150). Leucine is known as a ‘nutrient signal’ as it reduces muscle protein breakdown and further stimulates muscle protein synthesis(Reference Buse and Reid159–Reference Frexes-Steed, Lacy and Collins161). The ‘leucine signal’ stimulates the mTOR pathway, activity and enhances eIF4E-binding protein 1 phosphorylation and the association of eIF4E with eIF4G both in vitro and in vivo (Reference Anthony, Anthony and Kimball162–Reference Dardevet, Sornet and Balage164). These signalling pathways are known to become resistant to amino acid (i.e. leucine) stimulation in the case of chronic inflammation or oxidative stress(Reference Lang, Frost and Nairn134, Reference Marzani, Balage and Venien135, Reference Balage, Averous and Remond165), phenomena that also occur during immobilisation. Consequently, BCAA, and more particularly leucine, supplementation has been tested to limit muscle atrophy during immobilisation based on the hypothesis that increasing BCAA/leucine intake could overcome anabolic resistance. BCAA supplementation was compared with an equimolar mixture of non-EAA during a 14 d bed rest study in men; BCAA supplementation resulted in a 1·2 g/kg per d protein intake during the bed rest period but the protein turnover still decreased with both types of supplementation and muscle mass was not preserved by the nutritional intervention(Reference Stein, Donaldson and Leskiw166). Similarly, sixteen women were subjected to bed rest for 60 d; they were divided into a control group, which maintained ambulatory protein intake, and into a nutrition intervention group in which protein intake was increased from 1·2 to 1·6 g/kg per d. It is noticeable that this supplement included 0·18 g leucine/kg per d whereas recommended leucine consumption could be estimated at 0·05 g/kg per d based on the RDA. No effect of this treatment was observed(Reference Trappe, Creer and Slivka167, Reference Trappe, Creer and Minchev168). In women participating in a 60 d bed rest study, protein intake was increased during bed rest from 1 to 1·45 g/kg per d, with BCAA supplementation (3·6 g free leucine/d, 1·8 g free isoleucine/d, and 1·8 g free valine/d)(Reference Chopard, Lecunff and Danger169). This study used a transcriptomic approach and muscle mass was not studied but, interestingly, the counteracting effect of BCAA supplementation with leucine enrichment appeared limited since most of the genes up-regulated were moderately down-regulated after the nutrition intervention(Reference Chopard, Lecunff and Danger169). A leucine supplementation was also tested in immobilised animals. Young rats received 2·7 g leucine/kg per d for 3 d before immobilisation and during 7 d of immobilisation. Neither protein synthesis nor insulin sensitivity was modified with the supplemented diet, but skeletal muscle wasting was attenuated via inhibition of ubiquitin ligases(Reference Baptista, Leal and Artioli170), suggesting that leucine supplementation alone may be a more effective intervention than BCAA supplementation.
Supplementation with other amino acids has been tested in the prevention of immobilisation-induced atrophy. Oral supplementation with taurine was tested in immobilised rats as a countermeasure to muscle atrophy. The rationale of this study was that taurine has been proposed to protect muscle function in a variety of diseases through diverse mechanisms(Reference Huxtable171). This study demonstrated that taurine content was markedly decreased in immobilised muscles and that a high daily dose of taurine supplementation (5 g/kg per d) fully prevented this decrease but without preventing muscle atrophy(Reference Pierno, Liantonio and Camerino172). Because of assumed ergogenic effects, short-term creatine supplementation has also been proposed for limiting muscle atrophy during immobilisation. Young adults received 20 g creatine/d during muscle immobilisation in a cross-over trial; this resulted in a maintained lean tissue mass when compared with the placebo group(Reference Johnston, Burke and MacNeil173). Finally, cysteine supplementation showed a positive effect in limitation of immobilisation-induced atrophy, an effect attributed to the suppression of muscle protein ubiquitination and the normalisation of the ratio of reduced glutathione:oxidized glutathione (GSH:GSSG) in muscle(Reference Ikemoto, Nikawa and Kano174).
Altogether, these results suggest that increasing protein intake could be an efficient measure to counteract immobilisation-induced atrophy, most probably by overcoming the anabolism threshold. Free amino acid supplementation does not exhibit a clear effect in the maintenance of muscle mass since trials conducted with EAA and BCAA supplementation failed to preserve muscle mass during immobilisation. Additional data on taurine, creatine and cysteine supplementation are required to conclude definitively on their beneficial effects.
Nutritional interventions to reduce the anabolism threshold during immobilisation
Antioxidant and anti-inflammatory compounds
Oxidative stress and inflammation situations have been demonstrated to have a negative impact on muscle protein turnover, particularly by decreasing protein synthesis. As immobilisation is associated with local inflammation and oxidative stress, it may be postulated that these phenomena lead to an increased anabolism threshold, translating into the anabolic resistance of skeletal muscle to food intake. Antioxidant supplementation has been shown to be an effective countermeasure to fight oxidative stress in a wide variety of tissue types and conditions(Reference Powers, Kavazis and McClung110, Reference Cornelli175), and it is speculated that this might be an efficient approach to reduce muscle wasting associated with muscle disuse(Reference Servais, Letexier and Favier176). Similar results have been obtained with anti-inflammatory compounds(Reference Rieu, Magne and Savary-Auzeloux136) that improve muscle mass maintenance under atrophying conditions.
Particular attention has been given to polyphenols and their antioxidant properties. Among polyphenols, resveratrol, which is naturally found in grapes, berries, groundnuts and red wine, has been extensively studied for its health benefits. Resveratrol has been shown to mediate cardiomyocyte survival following simulated hypoxia–reperfusion by up-regulating both the antioxidant thioredoxin and the anti-apoptotic protein Bcl-2(Reference Brito, Simoes and Almeida177, Reference Das, Khan and Mukherjee178), thus underscoring its potential to act as both an antioxidant and anti-apoptotic compound. Recent data suggest that resveratrol induces the transcription of two key antioxidant enzymes, catalase(Reference Dani, Bonatto and Salvador179, Reference Kode, Rajendrasozhan and Caito180), and Mn superoxide dismutase (MnSOD)(Reference Robb, Winkelmolen and Visanji181–Reference Ryan, Jackson and Hao183). Adult rats (aged 6 months) were then supplemented with this polyphenol (12·5 mg/kg per d) for 3 weeks, including 2 weeks of muscle immobilisation. Despite a limitation in the functional decrements and the oxidative stress levels associated with muscle immobilisation, resveratrol was unable to suppress body weight loss and muscle mass loss(Reference Jackson, Ryan and Hao184). In another study, rats were fed a resveratrol-supplemented diet in a dose equivalent to 400 mg/kg per d before unloading and 2 weeks of muscle immobilisation. Resveratrol treatment significantly reduced disuse atrophy by 26 and 10 % without and with normalisation on body weight, respectively. This effect was attributed to the prevention of the decrease of the glutathione:glutathione disulfide ratio, a biomarker of oxidative stress(Reference Momken, Stevens and Bergouignan185). Translated into human doses, this represents a daily intake of 4·5 g/d, a dose for which potential side effects have to be studied before the relevance of its clinical use. These two studies tend to demonstrate that limitation of oxidative stress is able to prevent the metabolic, and muscle deconditioning caused by mechanical unloading only when a high dose is used. In another study, immobilised mice were fed a diet supplemented with 0·05 % tea catechins containing 81 % polyphenols (i.e. reported as animal food intake of 1·2–1·3 mg polyphenols, i.e. 46–50 mg/kg). The supplemented diet was consumed 14 d before immobilisation and during the 10 d of immobilisation. The supplemented diet significantly inhibited the decrease in tetanic force observed in the soleus muscle in response to immobilisation, but did not suppress muscle atrophy, therefore suggesting that tea catechins affect skeletal muscle function rather than skeletal mass, and prevent the decrease in muscle strength due to disuse(Reference Ota, Soga and Haramizu186).
Amelioration of disuse muscle atrophy following administration of various other antioxidants (i.e. vitamin E or soya protein extract) has been published(Reference Morris, Morris and Kennedy14, Reference Kondo, Miura and Nakagaki101, Reference Appell, Duarte and Soares187, Reference Arbogast, Smith and Matuszczak188). However, contradictory results have been obtained regarding the impact of vitamin E on muscle atrophy. Some authors highlighted no effect of vitamin E supplementation(Reference Koesterer, Dodd and Powers99), whereas others have shown that vitamin E reduced muscle atrophy(Reference Servais, Letexier and Favier176). Surprisingly in this study, its protective effect did not depend on its antioxidant function, but probably on a direct modulation of muscle proteolysis(Reference Servais, Letexier and Favier176). Dietary curcumin was used in rodents to prevent immobilisation-induced atrophy but without any effect on muscle mass atrophy(Reference Vazeille, Slimani and Claustre29, Reference Farid, Reid and Li189). Farid et al. (Reference Farid, Reid and Li189) reported that N-acetylcysteine did not suppress muscle atrophy or force decrease in tail-suspended mice, but suppressed considerably NF-κB activity, suggesting that the antioxidant may have a non-negligible but insufficient effect on pathways involved in disuse atrophy. Finally, a Cr supplement was used in hindlimb-suspended mice, based on the observation that nutritional supplementation with Cr has been shown to prevent weight loss and improve glucose tolerance in malnourished subjects on long-term total parenteral nutrition(Reference Freund, Atamian and Fischer190). Cr inhibited skeletal muscle atrophy and this was associated with the prevention of elevation of the UPS pathway, and better levels of Akt protein, suggesting a greater protein retention and hence contributing to the preservation of the muscle mass(Reference Dong, Hua and Zhao191). As immobilisation-induced atrophy is associated with impaired glucose metabolism, it may be postulated that this improvement in protein metabolism could result also from an improvement in glucose homeostasis.
Among anti-inflammatory compounds, dietary fish oil has been proposed to limit immobilisation-induced atrophy. Fish oil is naturally rich in long-chain n-3 fatty acids, namely DHA and EPA, which are known to have anti-inflammatory properties(Reference Fetterman and Zdanowicz192). n-3 Fatty acids are also able to enhance insulin-sensitive protein anabolism through the Akt/mTOR/S6K1(Reference Gingras, White and Chouinard193) pathway, which may help to reduce muscle anabolic resistance induced by immobilisation. In animals receiving 5 % fish oil, muscle mass atrophy following immobilisation was attenuated via mechanisms involving prevention of immobilisation-induced impairments of insulin signalling, the Akt/mTOR/S6K1 and muscle-specific ubiquitins ligases(Reference You, Park and Song57). However, these positive effects could have deleterious effects on the following recovery (see later).
Energy intake
Biolo et al. (Reference Biolo, Ciocchi and Stulle194) studied the interaction between muscle inactivity and energy restriction, based on the observation that bedridden individuals, elderly individuals and astronauts often combine muscle inactivity with a reduced energy intake that is below their energy expenditure. They submitted young healthy volunteers to bed rest for 14 d and low-energy diets containing about 80 % of total energy requirements. Bed rest associated with hypoenergetic nutrition led to the greatest wasting of lean body mass(Reference Biolo, Ciocchi and Stulle194). Completing these results in another study following the same protocol(Reference Biolo, Agostini and Simunic195), the same authors tested the hypothesis that energy intake in excess of requirements (i.e. a diet containing 1·2 times subjects' resting energy expenditure) could lead to fat deposition and thus accelerate inactivity-induced loss of lean mass by activating systemic inflammation, free radical production and antioxidant defences. Indeed, they demonstrated that this energy excess was associated with higher plasma C-reactive protein and myeloperoxidase concentrations (markers for inflammation). They concluded that excess energy can worsen muscle atrophy during bed rest, affecting whole-body protein turnover and systemic inflammation, respectively(Reference Biolo, Agostini and Simunic195). In conclusion, positive energy balance during inactivity is associated with higher muscle atrophy and with activation of systemic inflammation and of antioxidant defences. Optimising energy intake to the requirements may be a useful strategy for mitigating muscle loss during periods of chronic inactivity.
Nutritional intervention to improve muscle mass recovery
So far, most attention has been focused on preventing muscle mass loss in response to immobilisation. Indeed, most of the rare studies performed observed the impact of a specific type of nutrition given during the immobilisation phase on the subsequent recovery, i.e. after the removal of the immobilisation stimuli. Nevertheless, the recovery phase is also important. An efficient and quick recovery of muscle mass and strength is of key importance to the immobilised individual. Resistance training and reliable nutrition elicit increased muscle mass and strength(Reference Dreyer, Drummond and Pennings196) and EAA plus carbohydrates enhance muscle protein synthesis to a greater degree than either stimulus alone(Reference Dreyer, Drummond and Pennings196, Reference Fujita, Dreyer and Drummond197).
In human subjects, BCAA supplementation during 14 d of bed rest had little effect on protein synthesis or breakdown in the early recovery period beyond a small increase in N balance(Reference Stein, Leskiw and Schluter11, Reference Stein, Donaldson and Leskiw166, Reference Stein, Leskiw and Schluter198). Brooks et al. (Reference Brooks, Cloutier and Cadena155) found that EAA supplementation during 28 d bed rest and 14 d of recovery had no impact on leg lean mass and strength at the end of the recovery.
Effects regarding dietary interventions with anti-inflammatory compounds were less evident. Indeed, if supplementation in rats during immobilisation improved subsequent muscle mass recovery when animals were treated intraperitoneally with curcumin(Reference Vazeille, Slimani and Claustre29), dietary fish oil given during immobilisation blunted the following muscle mass recovery(Reference You, Park and Lee199). This effect was attributed to a suppression of Akt/S6K1 signalling and PGF2α content in the early phase of recovery, demonstrating that inflammatory processes are nevertheless required for the efficacy of muscle recovery(Reference You, Park and Lee199).
Ageing and bed rest: impact of nutritional interventions during disuse and following rehabilitation
Particular attention should be paid to prolonged periods of immobilisation during ageing. Indeed, elderly individuals are in a state of fragility when considering their physical activity. Ageing is characterised by a reduced physical activity mainly due to the age-related muscle mass loss, a condition referred to as sarcopenia. English & Paddon-Jones(Reference English and Paddon-Jones4) postulated in their review that sarcopenia could be worsened by catabolic states, as muscle mass atrophy could be followed by uncompleted muscle mass recoveries which, repeated throughout life, could result in a significant muscle mass loss. These uncompleted muscle mass recoveries have been well demonstrated in aged animals(Reference Magne, Savary-Auzeloux and Vazeille30, Reference Magne, Savary-Auzeloux and Migne31, Reference Chakravarthy, Davis and Booth200) and elderly individuals(Reference Suetta, Hvid and Justesen201) submitted to muscle immobilisation.
Decreased postabsorptive protein synthesis(Reference Kortebein, Ferrando and Lombeida2) and the presence of anabolic resistance in immobilised muscle have been demonstrated also during ageing(Reference Magne, Savary-Auzeloux and Migne31). As anabolic resistance has already been demonstrating naturally during ageing(Reference Dardevet, Sornet and Balage164, Reference Fujita, Glynn and Timmerman202–Reference Mosoni, Valluy and Serrurier204), it is likely that immobilisation may more deeply impair the blunted response of muscle protein synthesis to the stimulatory effect of food intake, hence contributing to the generation of a negative N balance and the resultant muscle mass atrophy.
The blunted EAA response of muscle protein synthesis was also demonstrated in aged humans submitted to bed rest, with a mechanism involving reduced mTORC1 signalling and amino acid transporter protein content(Reference Drummond, Dickinson and Fry205, Reference Hvid, Aagaard and Justesen206), suggesting that a blunted EAA stimulation of muscle protein synthesis may contribute to muscle loss with inactivity in older individuals. However, in older subjects subjected to a 10 d bed rest, an increase in the daily protein intake from 0·8 to 1·5 g/kg per d had no effect on muscle mass loss, but muscle strength was preserved(Reference Dillon, Sheffield-Moore and Paddon-Jones207). In another study, EAA supplementation was conducted in healthy elderly subjected to bed rest (45 g EAA daily between meals that provided the RDA for protein; 0·8 g/kg per d). After 10 d of bed rest, muscle protein synthesis was decreased by 30 % in controls but maintained in the supplementation group(Reference Ferrando, Paddon-Jones and Hays208). EAA also protected functional abilities such as floor transfer time and exhibited a trend for the protection of stair ascent power and standing plantar flexion but, surprisingly, no effect was reported on muscle mass. These two studies seem to demonstrate that increased protein intake combined with EAA supplementation could be a possible countermeasure for preserving muscle functionality, but inefficient in preserving muscle mass.
Dietary restriction (by 30 %) in old rats subjected to hindlimb suspension prevented the increases in proteasome activity and other UPS components and reduced muscle wasting(Reference Altun, Besche and Overkleeft209). As it has been well demonstrated that dietary restriction could reduce deleterious age-related changes(Reference Piper and Bartke210) including muscle wasting(Reference Altun, Bergman and Edstrom211) in elderly individuals consuming suitable amounts of dietary proteins. These studies highlight interesting nutritional interventions that could be used to cope with immobilisation-induced atrophy but have probably to be taken with care.
For reasons previously presented above, we tested free leucine supplementation during the recovery period in aged animals previously subjected to immobilisation. Indeed, free leucine has been demonstrated to be particularly efficient at stimulating muscle protein synthesis during ageing(Reference Dardevet, Sornet and Bayle212–Reference Rieu, Balage and Sornet216). Despite a positive effect on protein anabolism (i.e. free leucine supplementation stimulated muscle protein synthesis and decreased protein breakdown during the recovery period), no effect was observed on muscle mass(Reference Magne, Savary-Auzeloux and Migne31). We postulated that leucine, as supplemented in a free form, was absorbed rapidly and induced its anabolic ‘leucine signal’ for protein synthesis before there was sufficient availability of amino acids coming from dietary protein digestion(Reference Dardevet, Remond and Peyron51). Indeed, free leucine was added to casein proteins, which are slow-digested(Reference Boirie, Dangin and Gachon151, Reference Boirie, Gachon and Corny217–Reference Dangin, Boirie and Guillet219). Therefore, the duration of the protein synthesis could have been insufficient. This desynchronisation between the ‘leucine signal’ and the availability of substrates for protein synthesis could explain the insufficient positive N balance and the lack of protein accretion when free leucine is used(Reference Magne, Savary-Auzeloux and Migne31). In this study, we also tested a whey protein supplementation and a high-protein diet (26 % protein) to limit muscle mass loss by (1) using whey protein as a leucine-rich protein source and (2) combining the whey properties with a high-protein effect, i.e. inducing a higher and sustainable concentration of amino acids available for protein synthesis over the postprandial period. Indeed, whey proteins as fast-digested proteins are rapidly digested, leading to a large, albeit temporary, rise in plasma amino acid levels(Reference Boirie, Dangin and Gachon151) with a particular efficacy to induce a large positive N balance during ageing(Reference Pennings, Boirie and Senden220). A combination of whey and casein proteins clearly induced a high skeletal muscle protein synthesis and prolonged amino acid delivery to tissue(Reference Wilkinson, Tarnopolsky and MacDonald221). Both strategies were efficient in improving muscle mass recovery(Reference Magne, Savary-Auzeloux and Migne31).
As the effect of leucine may be mediated by its metabolites(Reference Mitch and Clark222), β-hydroxy-β-methylbutyrate (HMB), a leucine metabolite was given to old rats immobilised with a model of 14 d suspension. Although HMB could not fully prevent immobilisation-induced body weight or muscle loss in response to unloading, it: (1) prevented further force loss during reloading after unloading; (2) improved muscle mass in muscles that were reloaded after immobilisation; (3) blunted the extent of fibre atrophy in both fast and slow skeletal muscles in response to unloading and reloading after disuse; (4) significantly attenuated myonuclear apoptosis induced by immobilisation; (5) decreased the apoptotic index after reloading following immobilisation in muscles; and (6) reduced mitochondrial apoptotic signalling as indicated by lower levels of cleaved caspase-3, cleaved caspase-9, and Bax protein abundance in reloaded muscles(Reference Hao, Jackson and Wang223). Elderly submitted to bed rest and receiving tube feeding fed HMB supplementation for 2–4 weeks showed a reduced muscle breakdown; however, no direct measurement of muscle mass was assessed in this study(Reference Hsieh, Chow and Chang224).
Overall conclusion and future directions
Muscle mass loss is an inevitable issue in response to muscle disuse, a condition found in many situations (from bed rest imposed after injuries or trauma to non-pathological bed rest occurring with age-related decrease in physical activity). The physiopathological changes responsible for this immobilisation-induced atrophy are multiple, but the decreased protein turnover observed probably plays a central role. The anabolic resistance that emerges with immobilisation is probably due to the appearance of local inflammation and oxidative stress that increase the skeletal muscle anabolism threshold. Numerous nutritional interventions can be proposed as countermeasures to limit muscle mass atrophy during immobilisation and improve the subsequent recovery. Separate strategies were tested in young animals or humans to overcome the increased threshold of anabolism or to decrease this threshold to restore muscle sensitivity to food intake. However, to date, no consensual data provided have allowed us to develop an efficient strategy. Combining the two approaches may have a greater impact on muscle mass, for example by combining proteins/amino acids and antioxidants/anti-inflammatory compounds.
During immobilisation periods, one of the preferred approaches for nutritional support to limit muscle atrophy consists of protein/amino acid supplementation(s). However, such supplementations have demonstrated a modest impact on the atrophic response despite a strong effect on the increase in postprandial muscle protein anabolism. Clearly, much more data are needed regarding the beneficial effect of increased protein intake and creatine supplementation, which were based on evidence from sport nutrition. Alone, such dietary components have no positive impact on muscle mass but elicit an interesting effect on muscle function that should be studied in more depth. Moreover, some selected dietary components might decrease the anabolism threshold and when combined with well-known anabolic factors (i.e. leucine, whey) they might potentiate the postprandial muscle anabolism and thus limit disuse atrophy and/or improve following recovery. A key point is the question of timing as demonstrated with fish oil supplementation: depending of the timing of the n-3 NEFA consumption (i.e. during disuse or recovery) these compounds could either improve or worsen the muscle atrophy. Future research should focus on these aspects and test different combinations of supplementation of proteins/amino acids and antioxidants/anti-inflammatory compounds to boost muscle anabolism. Different timing targeting different biochemical events (i.e. to decrease oxidative stress/inflammation during immobilisation and to increase anabolism during the recovery process) should be also tested.
During ageing, muscle atrophy in response to disuse is a main issue since muscle atrophy episodes are followed by uncompleted muscle recovery which accelerates the development of sarcopenia. Dietary supplementation with proteins/amino acids seem inefficient to limit the atrophic processes, but demonstrated real positive effects in this particular population by improving the recovery of muscle mass after an acute catabolic state such as immobilisation. Such nutritional strategies could be more efficient when using antioxidants/anti-inflammatory compounds that can decrease the well-known low-grade inflammation associated with ageing and responsible for the blunted muscle response to food intake. Ongoing research may emphasise these data in well-designed clinical trials conducted on a long-term basis.
Acknowledgements
The authors would like to thank Hélène Lafarge for help in the management of the bibliography.
This research received no specific grant from any funded agency in the public, commercial or not-for-profit sectors.
H. M., I, S.-A., D. R. and D. D. wrote and approved the final manuscript.
The authors have declared no conflicts of interest.