Introduction
CVD and cognitive impairment are growing worldwide health concerns, particularly as populations age( Reference Yazdanyar and Newman 1 , Reference Drag and Bieliauskas 2 ). In 2006, the worldwide prevalence of Alzheimer's disease was estimated at 26·6 million and by 2050 this is predicted to quadruple( Reference Brookmeyer, Johnson and Ziegler-Graham 3 ). Increasing evidence suggests that CVD, the metabolic syndrome, hypertension, obesity and type 2 diabetes are associated with diminished cognitive functioning and an increase in all types of dementia( Reference Monsuez, Gesquière-Dando and Rivera 4 ). These cognitive changes may be mediated through compromises in the structural and functional integrity of cerebral blood vessels. Cognitive performance refers collectively to mental processes including attention, memory, language, problem solving and decision making. Understanding the mechanisms for regulating cognitive functions is important to reduce the impact of declining cognition in older adults. Interventions that slow or prevent this condition are valuable and have become a health priority( Reference Deary, Corley and Gow 5 ). One of the mechanisms by which cognitive performance can be improved and cognitive decline delayed may be through maintenance of blood vessel health and improvement in blood flow to the brain( Reference Sinn and Howe 6 , Reference Krestin, van der Lugt and Poels 7 ). Impaired vasodilatation contributes to reduced cognitive performance, due to poor peripheral and cerebral perfusion( Reference Silvestrini, Pasqualetti and Baruffaldi 8 ). Endothelial cells line blood vessels (including those in the brain); thus maintaining cerebral vascular function to ensure normal regulation of cerebral blood flow for the delivery of nutrients is essential to maintain endothelial cell integrity( Reference Iadecola and Davisson 9 ).
It has been hypothesised that inflammation may contribute to cognitive decline( Reference Arfanakis, Fleischman and Grisot 10 ) and to CVD processes( Reference Meigs, Hu and Rifai 11 ). This may be a result of endothelial dysfunction( Reference Bomboi, Castello and Cosentino 12 , Reference Singhal 13 ) associated with reduced NO bioavailability. NO is an important vasodilator, produced from l-arginine by endothelial NO synthase( Reference Lundberg and Gladwin 14 ). Early phases of atherosclerosis involve the adhesion of circulating monocytes to the endothelium (inner lining of blood vessel walls) and their migration to the intima layer. This is a complex disease process mediated by inflammatory responses that involve cytokine production and up-regulation of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin. An increase in inflammatory cytokines (for example, C-reactive protein (CRP) and IL-6) have been found to be independent predictors of CVD and type 2 diabetes( Reference Rana, Nieuwdorp and Jukema 15 ). The endothelium is crucial for the maintenance of vascular tone and vascular structure; endothelial dysfunction predisposes individuals to complications of atherosclerosis by increasing blood pressure and arterial stiffness, characterised by increased pulse-wave velocity and an increase in augmentation index. Endothelial function declines with age but is also adversely affected by hypertension, hyperglycaemia, dyslipidaemia and obesity, individually or collectively known as the metabolic syndrome( Reference Vita 16 ).
The principal energy source for the brain is glucose, which must be supplied continuously due to a limited storage capacity( Reference Benton 17 ). In addition, a range of nutrients and substrates including oxygen needs to be delivered via the blood( Reference Bourre 18 ); hence cerebral blood flow and substrate transport across the blood–brain barrier are primary determinants of brain function( Reference Benarroch 19 ). There is a growing interest in the role of nutrition in the causation and prevention of age-related cognitive decline and dementia; more research is needed to understand mechanisms for cognitive decline and possible delay.
As shown in Table 1, nuts contain a range of nutrients with potential health benefits including improved glucose control and insulin sensitivity( Reference Coates and Howe 20 , Reference Casas-Agustench, Bulló and Salas-Salvadó 21 ). Despite the high fat content of nuts, nut consumption has not been shown to increase body weight; instead it is associated with improved weight control( Reference Rana, Nieuwdorp and Jukema 15 , Reference Bes-Rastrollo, Sabaté and Gómez-Gracia 22 ). There is a substantial body of evidence demonstrating lipid-lowering effects of nut consumption( Reference Kris-Etherton and Griel 23 ) and large epidemiological studies have consistently revealed an association between frequent nut consumption and reduced incidence of CHD( Reference Kelly and Sabaté 24 ). A meta-analysis of thirteen intervention studies using walnuts( Reference Banel and Hu 25 ) and a pooled analysis of twenty-five intervention studies with a range of nuts indicated a consistent cholesterol-lowering effect( Reference Sabate, Oda and Ros 26 ). The analysis in the latter review revealed a 7·4 % reduction in LDL-cholesterol with a mean nut consumption of 67 g/d. Reductions in LDL-cholesterol were dose dependent, but not dependent on the type of nut consumed( Reference Sabate, Oda and Ros 26 ). The lipid-lowering effects may be attributed to the high content of unsaturated fat and fibre in nuts. Other bioactive nutrients in nuts may benefit glucoregulation( Reference Kendall, Josse and Esfahani 27 ), endothelial function, blood pressure control( Reference Casas-Agustench, López-Uriarte and Ros 28 ) and inflammation( Reference Casas-Agustench, Bulló and Salas-Salvadó 21 ). Studies have demonstrated that higher nut consumers are at a significantly lower risk of non-cardiovascular inflammatory disease mortality( Reference Gopinath, Buyken and Flood 29 ) and risk of developing type 2 diabetes( Reference Salas-Salvadó, Bulló and Babio 30 ) than low nut consumers. These benefits may be attributed to their nutrient profile; plant-derived n-3 fatty acids (α-linolenic acid; ALA) found in walnuts have been shown in clinical and epidemiological studies to improve inflammation, arterial compliance, insulin resistance, endothelial function and blood pressure( Reference Mozaffarian 31 – Reference Muramatsu, Yatsuya and Toyoshima 34 ). Nuts, especially consumed with their skin intact, have a significant amount of polyphenols( Reference Bolling, Chen and McKay 35 ). The results of many epidemiological studies suggest that the intake of polyphenol-rich foods has a beneficial effect on a large number of cardiovascular risk factors including high blood pressure and poor vascular function( Reference Lecour and Lamont 36 ). Polyphenols and vitamin E may have a role in modifying some of the inflammatory mediators( Reference Biesalski 37 , Reference Singh, Devaraj and Jialal 38 ) and be beneficial for cognitive performance( Reference Collie and Morley 39 , Reference Joshi and Praticò 40 ). Unsalted nuts contain high levels of K and Mg, making them a potential food for blood pressure control. However, nuts are commonly sold as a highly salted product and in this form can substantially increase the intake of Na, hence reducing their potential benefit. In addition, nuts contain fibre and l-arginine that has been shown to improve endothelial function( Reference Sánchez-Muniz 41 – Reference Lekakis, Papaioannou and Stamatelopoulos 44 ). Studies have investigated the impact of nuts on endothelial function( Reference Casas-Agustench, López-Uriarte and Ros 28 ); however, no study has taken the next step and considered whether nuts may have beneficial effects on cerebral vascular function and little research has been conducted on the impact of nut consumption on cognitive performance.
Table 1 Nutritional composition of nuts (per 100 g)

N/A, not available.
* Data from US Department of Agriculture, Agricultural Research Service( 140 ).
† Data from Kornsteiner et al. ( Reference Kornsteiner and Wagner 141 ).
‡ Data from Bolling et al. ( Reference Bolling, Chen and McKay 35 ).
§ Data from Yang et al. ( Reference Yang and Liu 142 ).
∥ Data from Tokuşoglu et al. ( Reference Tokuşoglu, Unal and Yemiş 143 ).
¶ Data from Blomhoff et al. ( Reference Blomhoff, Andersen and Carlsen 144 ).
Thus, unsalted nuts contain the precursor, key ingredients for cardiometabolic benefits needed to enhance blood vessel health, which may in turn improve cognitive function and limit cognitive decline as proposed in Fig. 1. Using a systematic search protocol, we reviewed the evidence for the effects of both tree and ground nuts on glucoregulation, blood pressure, arterial compliance, inflammation, endothelial vasodilator function and cognitive performance. As noted previously, there is a large body of consistent evidence demonstrating improvements in lipid regulation with nut consumption( Reference Banel and Hu 25 , Reference Sabate, Oda and Ros 26 ); hence this component has not been included in the present review.
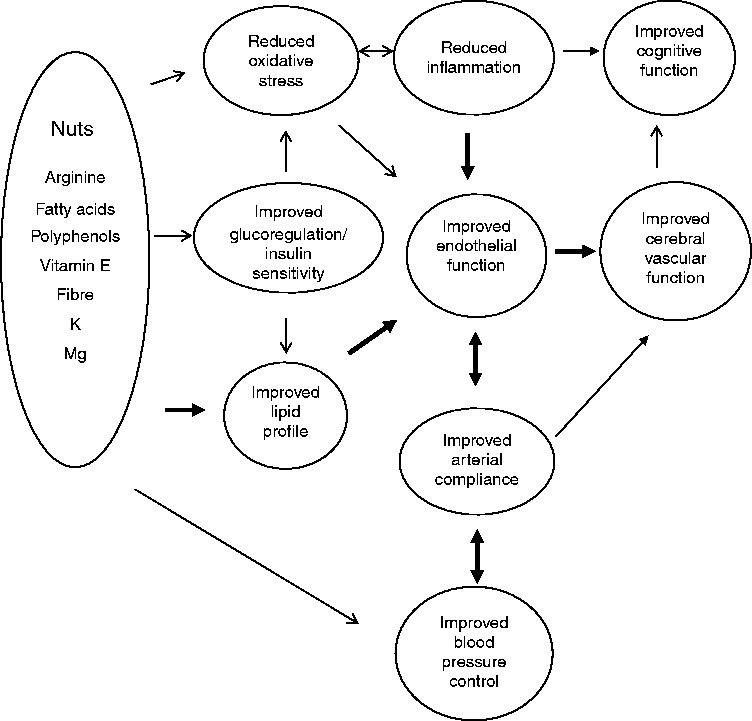
Fig. 1 Summary of potential effects of nutrients in nuts to improve cardiovascular risk factors (lipid profile, arterial compliance, glucoregulation, oxidative stress, blood pressure and inflammation) and consequent improvement in endothelial function and potential improvement in cerebral vascular function and hence cognitive performance. → , Weak evidence; → , strong evidence; ↔ , bi-directional effect; ↔ , strong bi-directional effect.
Methods
Selection of studies
Medline (via Ovid) and CINAHL (via Ebsco host) databases and the Cochrane Library were searched on 21 November 2012. Search terms used included MeSH (Medical Subject Headings) terms: ‘nuts’ OR ‘almond*’ OR ‘Brazil nut*’ OR ‘cashew*’ OR ‘hazelnut*’ OR ‘macadamia*’ OR ‘peanut*’ OR ‘pecan*’ OR ‘pistachio*’ OR ‘walnut*’ AND ‘endothel*’ OR ‘FMD’ OR ‘vascular*’ OR ‘blood pressure’ OR ‘arterial compliance’ OR ‘vasodilatation’ OR ‘glucose’ OR ‘insulin’ OR ‘inflam*’ OR ‘cognit*’. Limits included ‘human only’ and ‘English language’. In addition, reference lists from the publications identified by the database searches were also manually searched to identify other relevant articles that were not detected by the searches. Studies were included if they met the following criteria: intervention or epidemiological studies in human subjects. Intervention diets included at least one of the following nuts: almonds, cashews, hazelnuts, macadamias, groundnuts, pistachios, walnuts, pecans or Brazil nuts. Intervention studies included assessment of chronic nut consumption for a minimum period of 3 weeks, thereby assessing chronic changes. Published studies were required to be original research and evaluate the effects of nuts on at least one of the following in human subjects: glucoregulation, endothelial vasodilator function, arterial compliance, resting blood pressure, inflammation or cognitive performance. Studies were excluded if they were non-English-language papers, narrative reviews, systematic reviews, expert opinions, editorials, abstracts, letters to the editor, theses, or animal or in vitro studies. Weighted mean changes in glucoregulation, systolic and diastolic blood pressure, CRP, ICAM-1, VCAM-1 and endothelial vasodilator function were calculated for studies that reported data suitable for calculating a percentage change. Study sample size was used to weight the calculation of the overall mean percentage change across studies using STATA software (StataIC 11; StataCorp LP).
Results
The search revealed articles published between March 1993 and October 2013. Of the 4198 articles identified by all databases and nine articles identified from hand searching, 3019 were excluded as duplicates, 114 were excluded because of document type (review, note, letter, proceedings paper, or meeting abstract) and 837 were excluded because they did not assess endothelial function, blood pressure, inflammation, glucoregulation or cognitive performance in conjunction with nut consumption. Of the 237 articles screened (titles and abstracts), 166 were excluded because they did not meet the inclusion criteria. Therefore, seventy-one studies were included in the present review as shown in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart (Fig. 2). A total of forty-four studies evaluated blood pressure, thirty-two evaluated glucoregulation, thirty-one evaluated inflammatory markers, nine evaluated endothelial vasodilator function, two evaluated arterial compliance and four evaluated cognitive performance. A total of nine types of nut were used in these studies: almonds, Brazil nuts, cashews, hazelnuts, macadamias, groundnuts, pistachios, pecans and walnuts. The majority of studies examined walnuts, almonds and mixed/any nuts (Table 2).

Fig. 2 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart showing procedures used to identify studies investigating the effect of nuts on blood pressure, endothelial function, inflammation, arterial compliance, glucoregulation and cognition included in the systematic search.
Table 2 Number of measures of nut consumption on the effect on blood pressure, glucoregulation, inflammation, arterial compliance, endothelial function and cognition (some studies tested more than one type of nut)
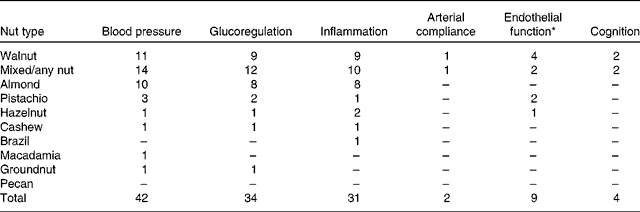
* Endothelial vasodilator function assessed by either flow-mediated dilatation or Endo-PAT device.
Most research measuring the effect of nut consumption on glucoregulation, blood pressure, inflammation, arterial compliance, endothelial vasodilator function and cognition has been performed with walnuts, mixed or non-specified nuts, almonds and pistachios, with only seven studies using groundnuts, hazelnuts, cashews, Brazil nuts or macadamias and no studies with pecans (Table 2). Studies are summarised in Tables 3–8 and are grouped according to outcomes, presented in order of efficacy (using mean percentage or blood pressure (mmHg) change where available). The following information was also extracted: author and year of publication, number, age and sex of the participants, type of individuals studied (i.e. healthy, hyperlipidaemic, high CHD risk, type 2 diabetes, overweight/obese or metabolic syndrome), study design, length of intervention, type and dose of nut, controls used and effect-size calculations where possible. Fig. 3 presents the number of outcome measures and the type of studies reflecting the level of evidence for these studies according to National Health and Medical Research Council guidelines( 45 ). Most intervention studies were randomised and controlled, providing greater evidence than uncontrolled or non-randomised trials.
Table 3 Studies measuring effect of nut consumption on glucoregulation

Glucose, fasting glucose; M, male; F, female; Dm, type 2 diabetes; Med diet, Mediterranean diet; ↓ , reduction; RR, relative risk; +, significant reduction; X-sect, cross-sectional; PREDIMED, PREvencion con DIeta MEDiterranea; N/A, not available; NS-G, no significant change; MESA, Multi-Ethnic Study of Atherosclerosis; NHANES, National Health and Nutrition Survey; RCT, randomised controlled trial; OO, olive oil; LF, low-fat; Met-S, metabolic syndrome; insulin, fasting insulin; HOMA, homeostatic model assessment of insulin resistance; NS, no significant difference; PCOS, polycystic ovary syndrome; HF, high-fat; LE, low-energy; CHO, carbohydrate; ↑ , increase; –, significant increase
* P≤ 0·05.
† Outcome (active v. control) for intervention studies.
‡ Mixed nuts = walnuts, almonds and hazelnuts.
§ Mixed nuts = almonds, pistachios, walnuts, groundnuts, hazelnuts, pecans, cashews and macadamias.
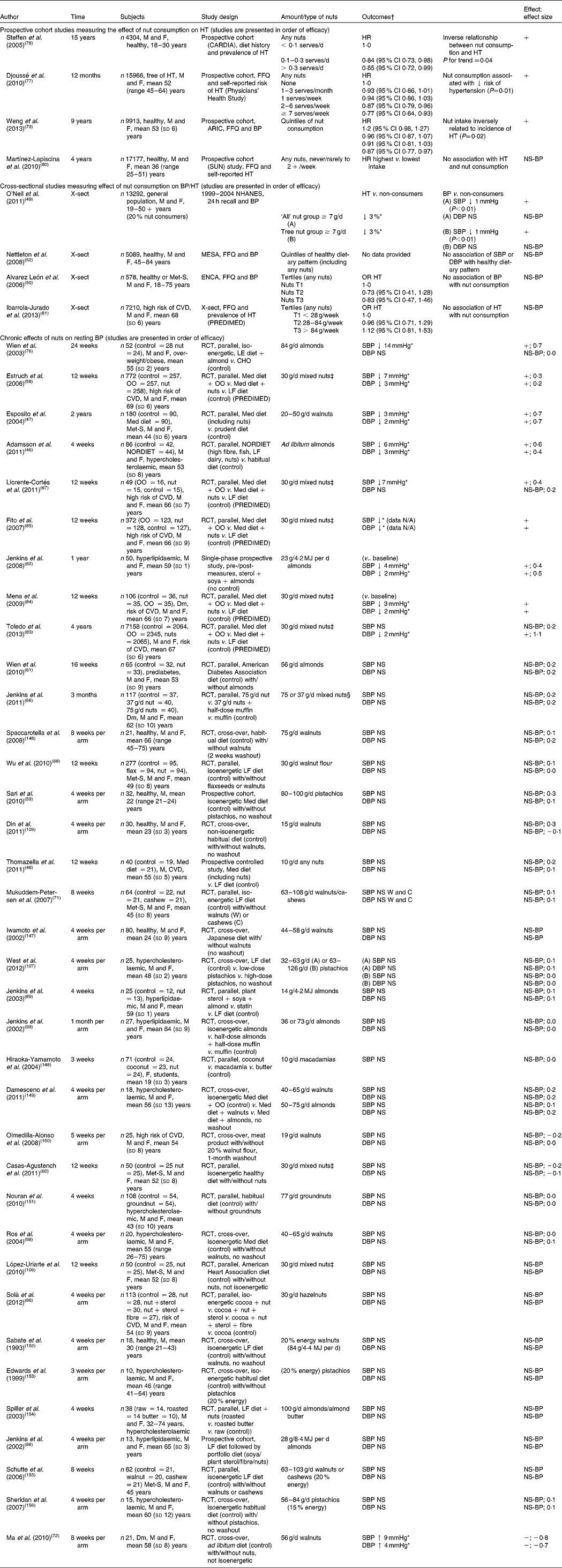
Table 4 Studies measuring effect of nut consumption on blood pressure (BP)
HT, hypertension; M, male; F, female; CARDIA, Coronary Artery Risk Development in Young Adults; HR, hazard ratio; +, significant reduction in BP/HT; ↓ , decrease; ARIC, Atherosclerosis Risk in Communities; NS-BP, no significant change in BP/HT; X-sect, cross-sectional; NHANES, National Health and Nutrition Survey; SBP, systolic BP; DBP, diastolic BP; NS, no significant difference; MESA, Multi-Ethnic Study of Atherosclerosis; ENCA, Canary Nutrition Survey; T1, T2, T3, tertiles; PREDIMED, PREvencion con DIeta MEDiterranea; RCT, randomised controlled trial; LE, low-energy; CHO, carbohydrate; OO, olive oil; Med diet, Mediterranean diet; N/A, not available; LF, low-fat; Met-S, metabolic syndrome; Dm, type 2 diabetes; ↑ , increase; –, significant increase in BP.
* P ≤ 0·05.
† Outcome (active v. control) for chronic studies.
‡ Mixed nuts = walnuts, almonds and hazelnuts.
§ Mixed nuts = almonds, pistachios, walnuts, groundnuts, hazelnuts, pecans, cashews and macadamias.
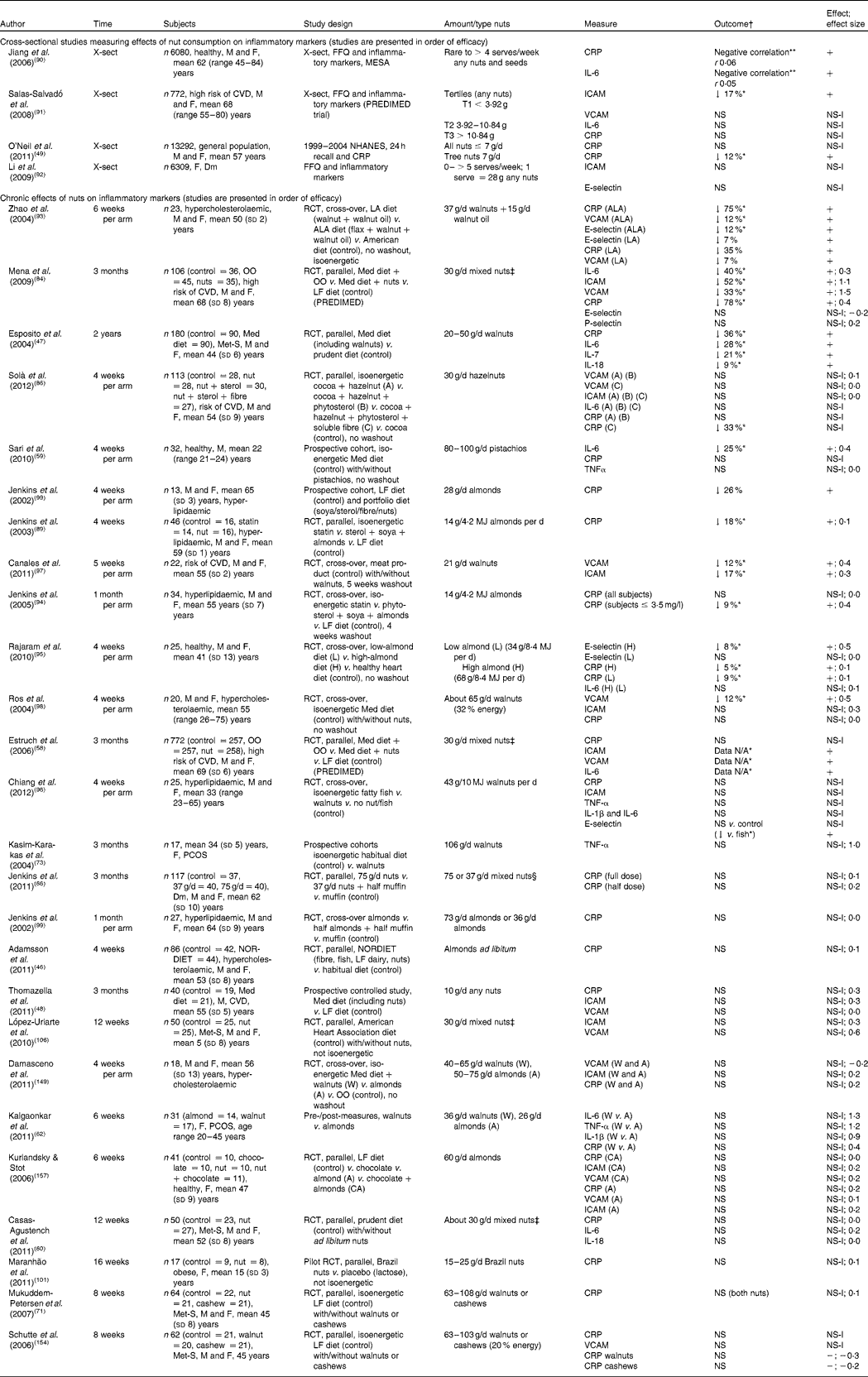
Table 5 Effects of nut consumption on inflammatory markers
X-sect, cross-sectional; M, male; F, female; MESA, Multi-Ethnic Study of Atherosclerosis; CRP, high-sensitivity C-reactive protein; +, significant decrease in inflammatory marker; PREDIMED, PREvencion con DIeta MEDiterranea; T1, T2, T3, tertiles; ICAM, intercellular adhesion molecule-1; VCAM, vascular cell adhesion molecule-1; ↓ , decrease; NS, no significant difference; NS-I, no significant change in inflammatory marker; NHANES, National Health and Nutrition Survey; Dm, type 2 diabetes; RCT, randomised controlled trial; LA, linoleic acid; ALA, α-linolenic acid; Med diet, Mediterranean diet; OO, olive oil; LF, low-fat; Met-S, metabolic syndrome; N/A, not available; PCOS, polycystic ovary syndrome; –, significant increase in inflammatory marker.
* P< 0·05, ** P< 0·01.
† Outcome (active v. control) for chronic studies.
‡ Mixed nuts = walnuts, almonds and hazelnuts.
§ Mixed nuts = almonds, pistachios, walnuts, groundnuts, hazelnuts, pecans, cashews and macadamias.
Table 6 Chronic effect of nut consumption on endothelial vasodilator function (studies are presented in order of efficacy)†
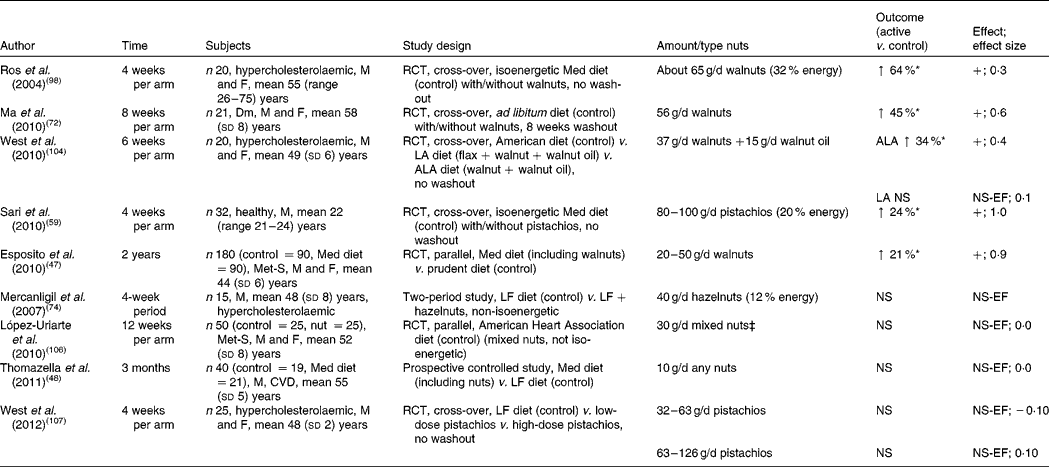
M, male; F, female; RCT, randomised controlled trial; Med diet, Mediterranean diet; ↑ , increase; +, significant increase in endothelial function; Dm, type 2 diabetes mellitus; LA, linoleic acid; ALA, α-linolenic acid; NS, not significant; NS-EF, no significant change in endothelial function; Met-S, metabolic syndrome; LF, low-fat.
* P< 0·05.
† Vasodilator function measured by flow-mediated dilatation, except López-Uriarte et al. (2010)( Reference López-Uriarte, Nogués and Saez 106 ), measured by Endo-PAT device.
‡ Mixed nuts = walnuts, almonds and hazelnuts.
Table 7 Effect of nut consumption on arterial compliance
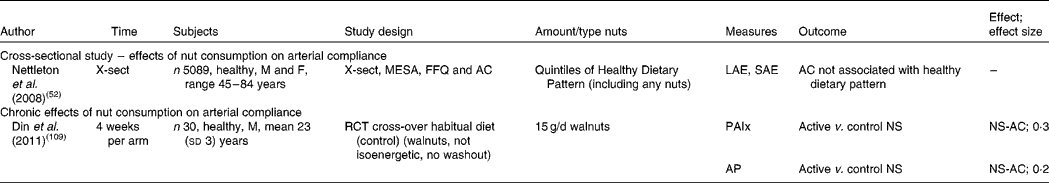
X-sect, cross-sectional; M, male; F, female; MESA, Multi-Ethnic Study of Atherosclerosis; AC, arterial compliance; LAE, large artery elasticity; SAE, small artery elasticity; RCT, randomised controlled trial; PAIx, peripheral augmentation index; AP, augmentation pressure; NS, no significant difference; NS-AC, no significant change in arterial compliance.
Table 8 Effects of nut consumption on cognitive function
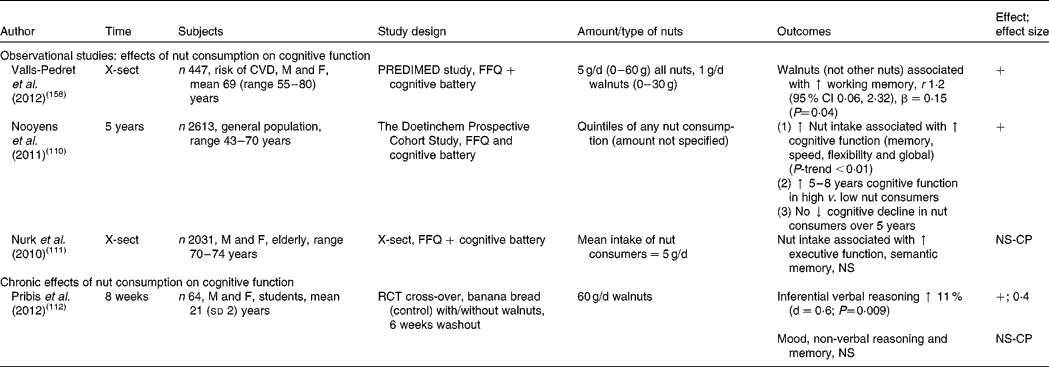
X-sect, cross-sectional; M, male; F, female; PREDIMED, PREvencion con DIeta MEDiterranea; ↑ , increase; +, significant increase in cognitive performance; ↓ , decrease; NS, no significant change; NS-CP, no significant change in cognitive performance; RCT, randomised controlled trial.
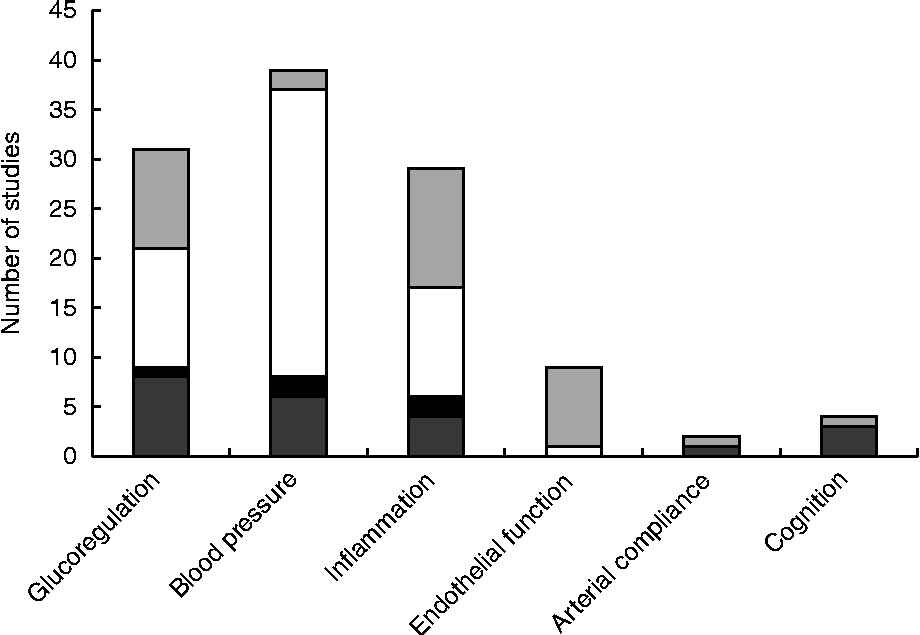
Fig. 3 Number of studies measuring effects of nut consumption on glucoregulation, blood pressure, inflammation, endothelial function, arterial compliance and cognition as epidemiological (▓), uncontrolled (■) or randomised controlled trials with primary (░) or secondary (□) outcomes.
Effects of nuts on glucoregulation
Details of studies measuring the effect of nut consumption on glucoregulation are reported in Table 3. A total of eight observational and twenty-four intervention trials evaluated the effects of chronic consumption of nuts on glucoregulation. Nuts consumed included walnuts, pistachios, groundnuts, almonds, cashews and mixed nuts, with amounts consumed ranging from 10 to 108 g/d (⅓ ounce to 4 ounces) (approximately 2–20 % of energy intake). The duration of consumption ranged from 4 weeks to 16 years. Intervention studies made comparisons with a healthy diet (fourteen studies), habitual diet (three studies), high-fat diet (one study) or other food products (five studies): muffins, pretzels, cereal bar, cheese or another type of nut. One study used no control. Of the studies, four compared habitual or healthy diets with intervention diets including nuts (NORDIET( Reference Adamsson, Reumark and Fredriksson 46 ) or a Mediterranean diet( Reference Esposito, Giugliano and Giugliano 47 , Reference Thomazella, Góes and Andrade 48 )).
Tree nuts were associated with a lower prevalence of fasting hyperglycaemia compared with non-nut consumers in the National Health and Nutrition Survey (NHANES) cohort study( Reference O'Neil, Keast and Nicklas 49 ). However, a healthy dietary pattern including nuts found no association with fasting glucose or insulin( Reference Alvarez León, Henríquez and Serra-Majem 50 ). It is possible that the amount of nuts consumed was insufficient to show benefits. Nut consumption has also been associated with a reduced risk of type 2 diabetes; evidence to support this comes from large epidemiological studies( Reference Jiang, Liu and Manson 51 – Reference Pan, Sun and Manson 54 ). The Nurses' Health Study demonstrated that consumption of nuts ( ≥ 5 times per week), peanut butter ( ≥ 5 times per week) or walnuts ( ≥ twice per week) was associated with a 24, 21 and 15 % lower risk, respectively, of developing type 2 diabetes( Reference Jiang, Liu and Manson 51 , Reference Pan, Sun and Manson 54 ) compared with those who never or rarely ate nuts; the effect was greatest in those of healthy body weight( Reference Jiang, Liu and Manson 51 ). In addition, the Shanghai Women's Health Study demonstrated that groundnut consumption was associated with a 22 % decreased risk of type 2 diabetes( Reference Villegas, Gao and Yang 55 ). The SUN Study demonstrated a 35 % reduced risk of type 2 diabetes with a Mediterranean diet including an unspecified quantity of nuts( Reference Martínez-González, de la Fuente-Arrillaga and Nunez-Cordoba 53 ). However, other components of the Mediterranean diet including olive oil and a high fibre intake may have also contributed to this outcome( Reference Biesalski 56 ). In contrast, the Iowa Women's Health Study did not find any association of consumption of foods high in vegetable fat (including nuts) and incidence of type 2 diabetes( Reference Meyer, Kushi and Jacobs 57 ), which may in part be due to the low mean intake of nuts in this cohort.
Clinical trials examining nut consumption and diabetes risk, glycaemic control or insulin resistance have suggested some beneficial effects. Some short-term intervention studies have shown benefits of nut consumption on glucose homeostasis( Reference Estruch, Martínez-González and Corella 58 , Reference Sari, Baltaci and Bagci 59 ) and insulin secretion( Reference Adamsson, Reumark and Fredriksson 46 , Reference Estruch, Martínez-González and Corella 58 , Reference Casas-Agustench, López-Uriarte and Bulló 60 , Reference Wien, Bleich and Raghuwanshi 61 ). The effects of nuts on insulin sensitivity are influenced strongly by changes in body weight, which may have accounted for the changes observed in one of these studies where participants reduced body weight with nut consumption. Longer intervention trials with Mediterranean diets supplemented daily with 20–50 g of walnuts or 30 g of mixed nuts (a mixture of walnuts, almonds and hazelnuts was used in the PREvencion con DIeta MEDiterranea (PREDIMED) trial as reported by Casas-Agustench et al. ( Reference Casas-Agustench, López-Uriarte and Bulló 21 )) resulted in a reduction in fasting glucose, insulin and improvement in insulin sensitivity (homeostatic model assessment of insulin resistance; HOMA)( Reference Esposito, Giugliano and Giugliano 47 ) and the incidence of type 2 diabetes by 52 % over 4 years( Reference Salas-Salvadó, Bulló and Babio 30 ). Benefits shown in studies with nuts included as part of the intervention diet (NORDIET( Reference Adamsson, Reumark and Fredriksson 46 ) or Mediterranean diet( Reference Esposito, Giugliano and Giugliano 47 )) may have been partly due to other components of these diets( Reference Kalgaonkar, Almario and Gurusinghe 62 ). Other studies have not shown benefits; consumption of pistachios, almonds, walnuts and a Mediterranean diet (supplemented with 10 g nuts/d) revealed no effect on fasting glucose or insulin( Reference Thomazella, Góes and Andrade 48 , Reference Zaveri and Drummond 63 – Reference Wien, Kandeel and Sabate 76 ). One study that failed to achieve an improvement in insulin sensitivity supplemented participants' diets with 100 g almonds/d for 4 weeks. In this study there was a significant weight gain, which may have masked any benefit on insulin control( Reference Lovejoy, Most and Lefevre 65 ). Unexpected increases in plasma glucose (but not insulin) were observed with walnut and cashew consumption in women with polycystic ovary syndrome and adults with the metabolic syndrome, respectively( Reference Mukuddem-Petersen, Stonehouse Oosthuizen and Jerling 71 , Reference Kasim-Karakas, Almario and Gregory 73 ). Other studies investigated HbA1c in individuals with type 2 diabetes and found that 28 g walnuts/d and 36 g almonds/d reduced HbA1c by 4 %( Reference Kalgaonkar, Almario and Gurusinghe 62 , Reference Cohen and Johnston 64 ). However, in other individuals, HbA1c did not change with 37–75 g mixed nuts/d( Reference Jenkins, Srichaikul and Banach 66 ), 30–50 g walnuts/d( Reference Tapsell, Gillen and Patch 69 , Reference Tapsell, Batterham and Teuss 70 , Reference Ma, Njike and Millet 72 ) or 57–112 g almonds/d (for 4 weeks)( Reference Lovejoy, Most and Lefevre 65 ). The lack of effect in the latter study may have been due to the short intervention time. Whilst epidemiological studies suggest an association of nut consumption with improvement in glucoregulation and diabetes risk, not all evidence from randomised controlled trials is supportive. Some inconsistencies in findings may be attributed to variations in the number or health status of the study participants, length of trial, or the dose of nuts used.
Weighted mean changes in glucoregulation indicate significant reductions in fasting insulin and HOMA scores of 14 (95 % CI − 24, − 4·5) % and 34 (95 % CI − 49, − 19) %, respectively, with small non-significant reductions of 2·8 (95 % CI − 6·9, 1·3) % and 1 (95 % CI − 3, 0·9) % for fasting glucose and HbA1c, respectively. This indicates positive effects of nut consumption on the most widely accepted markers of glucoregulation. Overall, there is considerable evidence of benefits of nut consumption for glycaemic control and insulin sensitivity observed after 4–6 weeks of consumption. However, inconsistencies make it difficult to reach precise conclusions on the role of nuts. The target population, dose and length of consumption (particularly to observe changes in HbA1c) need to be further considered so that targeted advice can be provided to consumers.
Effects of nut consumption on blood pressure
Studies measuring the effect of nut consumption on blood pressure are found in Table 4. Nuts consumed included walnuts, pistachios, groundnuts, almonds, cashews, hazelnuts, macadamias and mixed nuts in different forms including oil, whole nuts and nut flour added to baked goods. As with many studies using whole-food products, participant blinding was not possible. Amounts consumed ranged from 10 to 108 g/d (⅓ ounce to 4 ounces/d) (approximately 2–20 % of energy intake). The length of consumption ranged from 3 weeks to 2 years. Whilst there were thirty-six intervention trials that reported on the effect of chronic consumption of nuts on blood pressure, most measured blood pressure as a secondary outcome. Comparisons were made with a healthy diet (sixteen studies), habitual diet (seven studies) or other food products including butter, muffins, processed meat, olive oil and cocoa. Of the studies, four compared habitual or healthy diets with intervention diets including nuts (NORDIET( Reference Adamsson, Reumark and Fredriksson 46 ) or a Mediterranean diet( Reference Esposito, Giugliano and Giugliano 47 , Reference Thomazella, Góes and Andrade 48 )); only one of the studies reported controlling for salt intake( Reference Adamsson, Reumark and Fredriksson 46 ). The remaining studies used control diets with unsalted nuts added as the intervention but overall dietary salt intake was not specified. Four prospective cohort studies measured blood pressure or incidence of hypertension in participants consuming nuts. The Physicians' Study demonstrated a significant reduction in self-reported hypertension after 12 months in those consuming nuts ≥ twice per week (hazard ratio 0·87; 95 % CI 0·79, 0·96) and greatest reduction with consumption ≥ 7 times per week (hazard ratio 0·77; 95 % CI 0·64, 0·93)( Reference Djoussé, Gaziano and Kase 77 ). However, salt intake and changes in weight were not accounted for, which could have affected outcomes observed. The Coronary Artery Risk Development in Young Adults (CARDIA) Study demonstrated an inverse relationship between nut consumption and prevalence of hypertension despite those classified as the highest consumers only consuming nuts ≥ 2 times per week (hazard ratio 0·85; 95 % CI 0·64, 0·93)( Reference Steffen, Kroenke and Yu 78 ). In support of this, the Atherosclerosis Risk in Communities (ARIC) Study also reported that nut consumption was inversely related to a reduced risk of hypertension; those who consumed approximately two serves of nuts per week were at a lower risk of hypertension than those who rarely or never consumed nuts (hazard ratio 0·87; 95 % CI 0·77, 0·97)( Reference Weng, Steffen and Szklo 79 ). In contrast, the SUN Study demonstrated no association between hypertension and nut consumption after a 4-year follow-up( Reference Martínez-Lapiscina, Pimenta and Beunza 80 ). However, the young educated adult sample in this study is less likely to demonstrate improvements in blood pressure with a dietary intervention than older individuals who are more likely to have higher blood pressure.
In all, four cross-sectional studies were identified comparing blood pressure or prevalence of hypertension in nut consumers with low-/non-nut consumers. The National Health and Nutrition Survey (NHANES) observed a general population and found a 3 % lower risk of hypertension and 1 mmHg reduction in systolic and diastolic blood pressure in nut consumers( Reference O'Neil, Keast and Nicklas 49 ). The Canary Nutrition Survey demonstrated a trend for reduced prevalence of hypertension with higher nut consumption but this did not reach significance( Reference Alvarez León, Henríquez and Serra-Majem 50 ). The Multi-Ethnic Study of Atherosclerosis (MESA) in Spain did not find an association with a healthy dietary pattern (incorporating an undetermined quantity of nuts) and blood pressure( Reference Nettleton, Schulze and Jiang 52 ). The authors suggest that routinely assessed blood pressure may have increased risk factor awareness, thereby attenuating associations with dietary intake. No association was found with hypertension and nut consumption in participants with a high risk of CVD( Reference Ibarrola-Jurado, Bulló and Guasch-Ferré 81 ). However, 90 % of the participants were hypertensive which may have made it difficult to demonstrate a relationship in this population. It is more difficult to account for health benefits from an individual food with observational studies; hence intervention studies are important to isolate effects.
Significant reductions in blood pressure were observed in nine intervention studies( Reference Adamsson, Reumark and Fredriksson 46 , Reference Esposito, Giugliano and Giugliano 47 , Reference Estruch, Martínez-González and Corella 58 , Reference Llorente-Cortés, Estruch and Mena 67 , Reference Wien, Kandeel and Sabate 76 , Reference Jenkins, Vidgen and Trautwein 82 – Reference Mena, Casas and Lamuela-Raventós 84 ). Effect sizes could be calculated in seven of these and were small to large, ranging between 0·2 and 1·1. A substantial reduction in systolic blood pressure (14 mmHg) was reported in participants who were overweight or obese and mildly hypertensive consuming a diet containing 84 g almonds/d for 24 weeks, compared with an isoenergetic high-carbohydrate diet( Reference Wien, Kandeel and Sabate 76 ), with some participants reducing or eliminating the use of antihypertensive medications during the duration of the study. A weight reduction of 7 % (BMI reduction of 2·5 kg/mReference Drag and Bieliauskas 2 ) was also observed in the participants consuming nuts compared with the control, despite the two groups being prescribed isoenergetic diets which would have accounted for at least some of the reduction in blood pressure observed( Reference Wien, Kandeel and Sabate 76 ). The PREDIMED Study tested the consumption of a Mediterranean diet which included 30 g mixed nuts/d compared with a Mediterranean diet devoid of nuts( Reference Estruch, Martínez-González and Corella 58 ). The study found a significant reduction in systolic and diastolic blood pressure of 7 and 3 mmHg, respectively. This study used a large cohort of 772 participants; subgroups of this study with 49–106 participants also reported similar reductions in blood pressure( Reference Llorente-Cortés, Estruch and Mena 67 , Reference Mena, Casas and Lamuela-Raventós 84 , Reference Fito, Marrugat and Garcia-Arellano 85 ). A larger cohort of the PREDIMED Trial found only a significant reduction in diastolic blood pressure( Reference Toledo, Hu and Estruch 83 ). The NORDIET included nuts as part of the intervention diet( Reference Adamsson, Reumark and Fredriksson 46 ), and reductions were demonstrated in systolic and diastolic blood pressure of 6 mmHg (effect size 0·6) and 2 mmHg (effect size 0·3), respectively. Almonds (23 g/d) consumed of as part of a portfolio diet with plant sterols and soya for 1 year demonstrated a reduction in systolic and diastolic blood pressure in a single-phase prospective study( Reference Jenkins, Vidgen and Trautwein 82 ). However, as no control group was used, it is possible that the regular clinic visits in this study increased participant awareness of hypertension as a CVD risk factor and other behaviour change may have contributed to the reduction in blood pressure in addition to the almond intervention( Reference Jenkins, Vidgen and Trautwein 82 ); without a control group this could not be determined. Consumption of a Mediterranean diet including 20–50 g walnuts/d compared with a prudent diet demonstrated reductions in systolic blood pressure of 3 mmHg (effect size 0·7) and in diastolic blood pressure of 2 mmHg (effect size 0·7)( Reference Esposito, Giugliano and Giugliano 47 ).
The majority of the remaining studies demonstrated either small blood pressure reductions which did not reach significance or no change. A reduction in systolic and diastolic blood pressure was observed with consumption of 40 g hazelnuts/d for 4 weeks from baseline; however, this was not significantly different from the reduction observed with cocoa used as the control( Reference Solà, Valls and Godàs 86 ). Inclusion of a control food that is not likely to change inflammation or endothelial function may have been a better choice to determine the effects attributable to hazelnuts( Reference Davison, Berry and Misan 87 ). An ad libitum diet with 56 g walnuts/d consumed by participants with type 2 diabetes for 8 weeks showed an increase in systolic (effect size − 0·8) and diastolic (effect size − 0·7) blood pressure. This unexpected result was from the only study that demonstrated a significant increase in blood pressure( Reference Ma, Njike and Millet 72 ). The authors were not able to determine a reason for this increase in blood pressure. However, other factors in the diet such as Na consumption may have contributed to the blood pressure elevation (despite being prescribed unsalted nuts); Na intake was not reported or controlled for in this study. Interventions using a portfolio diet( Reference Jenkins, Josse and Leiter 88 , Reference Jenkins, Lapsley and Trautwein 89 ), NORDIET( Reference Adamsson, Reumark and Fredriksson 46 ) or Mediterranean diet( Reference Esposito, Giugliano and Giugliano 47 , Reference Thomazella, Góes and Andrade 48 ) contained foods other than nuts which may also have been beneficial for improvements in blood pressure, making it difficult to tease out the effects of nuts alone. In contrast, Mediterranean diets in which mixed nuts( Reference Estruch, Martínez-González and Corella 58 , Reference Llorente-Cortés, Estruch and Mena 67 ) replaced olive oil demonstrated improvements in blood pressure, indicating there may be some beneficial effect of nuts above that of other components of the Mediterranean diet. The largest effects of nuts on blood pressure were seen in participants with the metabolic syndrome or other risk factor for CVD, consuming 30–84 g of almonds, walnuts or mixed nuts/d for 4 weeks to 2 years. Significant reductions of 3–14 and 2–3 mmHg were observed in systolic and diastolic blood pressure, respectively( Reference Adamsson, Reumark and Fredriksson 46 , Reference Esposito, Giugliano and Giugliano 47 , Reference Estruch, Martínez-González and Corella 58 , Reference Llorente-Cortés, Estruch and Mena 67 , Reference Wien, Kandeel and Sabate 76 , Reference Mena, Casas and Lamuela-Raventós 84 , Reference Fito, Marrugat and Garcia-Arellano 85 ). Only two of thirty-six studies measured resting blood pressure as a primary outcome, so the remaining studies may not have been powered to detect small changes. In eight of the nine studies demonstrating blood pressure reductions, nuts were consumed for extended periods of between 12 weeks to 2 years. Most studies demonstrated no beneficial effect on blood pressure when nuts were consumed for shorter periods (3–12 weeks). This suggests a benefit of nut consumption only after an extended period of time as indicated with observational studies where habitual nut consumption was associated with reduced blood pressure or reduced prevalence of hypertension.
Weighted mean changes in blood pressure were calculated for twenty-four of the thirty-six intervention studies; systolic and diastolic pressure were significantly reduced by 0·73 (95 % CI − 1·3, − 0·2) % and 0·75 (95 % CI − 1·1, 0·4) %, respectively (see Table 9). Improvements in blood pressure control were observed particularly when nuts were consumed regularly for extended periods of time. Although the effect of nut consumption on blood pressure is small, this may still be clinically meaningful especially when used with other lifestyle measures.
Table 9 Weighted mean percentage changes in blood pressure, inflammatory markers, endothelial function and glucoregulation with nut consumption

BP, blood pressure; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; HOMA, homeostatic model assessment of insulin resistance.
* Statistically significant (P< 0·05).
† Endothelial vasodilator function assessed by either flow-mediated dilatation or Endo-PAT device.
Effects of nut consumption on inflammatory markers
Studies measuring the effect of nut consumption on inflammatory markers are found in Table 5. The most commonly measured inflammatory marker was CRP, reported in twenty-seven of the thirty-one studies. Other inflammatory markers measured included TNF-α, interleukins (IL-1, IL-1β, IL-6, IL-7 and IL-18) and cellular adhesion molecules (ICAM-1, VCAM-1 and E-selectin). We identified four cross-sectional studies and twenty-seven intervention trials measuring inflammatory markers with nut consumption. Of the intervention studies, eleven compared nuts with a healthy diet (low-fat or Mediterranean diet), five with a Western, American or habitual diet, six studies compared nut consumption with another food product (meat, cocoa, lactose or olive oil), one study compared two types of nut and one study was a single intervention using pre- and post-measures with no control or comparator food. The range of nuts used included almonds, walnuts, mixed nuts, Brazil nuts, cashews, pistachios and hazelnuts. The amounts ranged from 10 to 103 g (⅓ ounce to 4 ounces) of nuts per d (approximately 5–25 % of energy intake) for 4 weeks to 2 years. To date, only tree nuts have been tested for effects on inflammatory markers with chronic nut consumption.
In three of the four cross-sectional studies, nut consumption was associated with lower concentrations of the inflammatory markers CRP, IL-6 or ICAM. The Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated an inverse association between frequency of consumption of nuts and seeds and serum CRP and IL-6 levels( Reference Jiang, Jacobs and Mayer-Davis 90 ). This association was moderately attenuated by additional adjustment for BMI. In two other studies, a Mediterranean diet pattern (PREDIMED study) or an American diet including nuts was inversely associated with anti-inflammatory markers( Reference Salas-Salvadó, Casas-Agustench and Murphy 91 ). Surprisingly, a large study (6309 women with diabetes), which categorised the largest nut consumption as ≥ 5 serves per week (1 serve = 28 g nuts or 18 g peanut butter) showed no association with inflammatory markers( Reference Li, Brennan and Wedick 92 ).
A total of twelve intervention studies demonstrated significant reductions (5–75 %) in inflammatory markers with nut consumption with a variety of nuts. Consumption of 21–100 g of walnuts, almonds, hazelnuts, pistachios or mixed nuts per d for 4 weeks to 2 years in healthy or hypercholesterolaemic participants or those at high risk of CVD resulted in significant reductions of CRP (5–75 %)( Reference Esposito, Giugliano and Giugliano 47 , Reference Mena, Casas and Lamuela-Raventós 84 , Reference Solà, Valls and Godàs 86 , Reference Jenkins, Josse and Leiter 88 , Reference Jenkins, Lapsley and Trautwein 89 , Reference Zhao, Etherton and Martin 93 – Reference Rajaram, Connell and Sabaté 95 ) or other inflammatory markers (ICAM, VCAM, E-selectin and interleukins) (7–28 %)( Reference Esposito, Giugliano and Giugliano 47 , Reference Estruch, Martínez-González and Corella 58 , Reference Sari, Baltaci and Bagci 59 , Reference Mena, Casas and Lamuela-Raventós 84 , Reference Zhao, Etherton and Martin 93 , Reference Rajaram, Connell and Sabaté 95 – Reference Ros, Núñez and Pérez-Heras 98 ). A study incorporating walnuts (37 g/d) plus walnut oil (15 g/d) or walnuts and walnut oil plus flax seed as an additional source of ALA demonstrated anti-inflammatory effects compared with an American diet in hypercholesterolaemic individuals( Reference Zhao, Etherton and Martin 93 ). The vascular adhesion molecules ICAM-1, VCAM-1 as well as CRP were all reduced significantly, with a dose–response effect found for ALA in the diet with a 75 % reduction in CRP. Participants who consumed 20–50 g walnuts/d for 2 years as part of a Mediterranean diet demonstrated a reduction in CRP (36 %) and interleukins IL-6, IL-7 and IL-18 (9–28 %) when compared with a prudent diet( Reference Esposito, Giugliano and Giugliano 47 ). Mediterranean diets in which walnuts (about 65 g/d) or mixed nuts (30 g/d) replaced olive oil demonstrated improvements in one or more of the inflammatory markers CRP, ICAM-1, VCAM-1 and IL-6( Reference Estruch, Martínez-González and Corella 58 , Reference Mena, Casas and Lamuela-Raventós 84 , Reference Ros, Núñez and Pérez-Heras 98 ). A reduction in CRP was demonstrated in four studies with a portfolio diet containing either almonds( Reference Jenkins, Lapsley and Trautwein 89 , Reference Jenkins, Li and Josse 94 , Reference Jenkins, Kendall and Marchie 99 ) (14–30 g/d) or hazelnuts (30 g/d)( Reference Solà, Valls and Godàs 86 ) consumed for 4 weeks; one of these studies found an reduction equivalent to that observed with statin intake in the same individuals( Reference Jenkins, Lapsley and Trautwein 89 ). Beneficial improvements in inflammation observed in interventions which contained foods in addition to nuts (portfolio diet( Reference Jenkins, Josse and Leiter 88 , Reference Jenkins, Lapsley and Trautwein 89 ) or Mediterranean diet( Reference Esposito, Giugliano and Giugliano 47 , Reference Thomazella, Góes and Andrade 48 )) may have been attributable to these other components. In contrast, Mediterranean diets in which walnuts( Reference Canales, Sánchez-Muniz and Bastida 97 ), mixed nuts( Reference Estruch, Martínez-González and Corella 58 , Reference Llorente-Cortés, Estruch and Mena 67 ) or pistachios( Reference Sari, Baltaci and Bagci 59 ) replaced olive oil demonstrated improvements in one or more of the inflammatory markers CRP, ICAM-1,VCAM-1 and IL-6, indicating that there may be some beneficial effect of nuts above that of other components of the Mediterranean diet. One study observed a reduction in CRP with a portfolio diet containing almonds only when participants with baseline CRP of ≤ 3·5 mg/l were excluded from analysis( Reference Jenkins, Li and Josse 94 ). (CRP levels ≥ 3·5 mg/l reflect acute inflammation associated with infection or acute illness that would mask any potential effects of nuts on chronic inflammation.)( Reference Gotto 100 ) A 25 % reduction in IL-6 was observed with a relatively large dose (80–100 g/d) of pistachios consumed for 4 weeks( Reference Sari, Baltaci and Bagci 59 ). Consumption of a high-almond diet (68 g/d per 2000 kcal or 8368 kJ) and a low-almond diet (34 g/d per 2000 kcal or 8368 kJ) for 4 weeks significantly decreased CRP compared with an isoenergetic control diet in healthy men and women( Reference Rajaram, Connell and Sabaté 95 ); E-selectin (a marker of endothelial inflammation) was significantly lower in the higher-almond group than control. No dose–response relationship was observed with either inflammatory marker in this study. In participants at risk of CVD, statistically significant reductions of the cellular adhesion molecules ICAM-1 (effect size 0·3) and VCAM-1 (effect size 0·4) were demonstrated with relatively low doses (21 g/d) of walnuts added to a meat product compared with the meat product without walnuts. Despite little evidence for the magnitude of nut dose influencing inflammation, it is possible that there is a minimum dose required since no studies using < 30 g/d demonstrated benefits.
In fifteen studies no significant changes in inflammatory markers were demonstrated, although most of these demonstrated small reductions. A recent three-arm study compared fatty fish v. walnuts v. a fish-/nut-free diet (control). No significant changes were found between the walnut and the control diets but E-selectin was reduced with the walnut intervention compared with the fish intervention( Reference Chiang, Haddad and Rajaram 96 ). Consumption of 26 g almonds/d or 36 g walnuts/d for 6 weeks led to a 19 % reduction in IL-6 with the almonds and 20 % reduction in TNF-α with the walnuts compared with baseline, but this did not reach significance. In addition, two studies with obese individuals demonstrated small but non-significant improvements in CRP( Reference Maranhão, Kraemer-Aguiar and de Oliveira 101 ) and IL-6( Reference Casas-Agustench, López-Uriarte and Bulló 60 ) with Brazil nut and mixed nut consumption, respectively. Suggested reasons for small but non-significant reductions in inflammatory markers were recruitment of healthy individuals who may only demonstrate limited improvements and diurnal effects of IL-6 that are more difficult to detect than other markers. One study with obese individuals demonstrated small but non-significant improvements in CRP( Reference Maranhão, Kraemer-Aguiar and de Oliveira 101 ). Increased central adiposity and body weight are associated with increased CRP levels and adipose pro-inflammatory cytokines including IL-6( Reference Mathieu, Lemieux and Després 102 ). It is possible that these individuals may not demonstrate improvements in inflammatory markers with a dietary intervention without weight loss.
The calculated weighted mean changes for all studies where data revealed reductions in ICAM-1, VCAM-1 and CRP were 8·6 (95 % CI − 20·5, 3·3) %, 5·8 (95 % CI − 14·1, 2·5) % and 12 (95 % CI − 23·6, − 0·3) %, respectively (see Table 9). In summary, nut consumption has the potential to improve inflammatory markers, particularly with doses of 30 g or greater. This is in line with a health claim for nuts first established by the US Food and Drug Administration (FDA) in 2003; scientific evidence suggests that eating 42 g (1·5 ounces) of most nuts per d (as part of an overall healthy diet) may be able to reduce the risk of heart disease( 103 ).
Effects of nut consumption on endothelial vasodilator function
Studies measuring the effect of nut consumption on endothelial vasodilator function are found in Table 6. In nine intervention studies the effect of nut consumption on endothelial vasodilator function (using either flow-mediated dilatation or Endo-PAT device) was measured, with the dose of nuts ranging from 10 to 100 g/d for periods ranging from 4 to 12 weeks. Of the nine studies, five demonstrated a significant effect.
Endothelial function was significantly improved (24–64 %) in healthy and hypercholesterolaemic participants who consumed 37–100 g of walnuts or pistachios per d for 4–8 weeks( Reference Sari, Baltaci and Bagci 59 , Reference Ma, Njike and Millet 72 , Reference Ros, Núñez and Pérez-Heras 98 , Reference West, Holub and Kris-Etherton 104 ). A Mediterranean diet supplemented with 65 g walnuts/d substituted for olive oil significantly improved vasodilation by 64 % in hypercholesterolaemic adults( Reference Ros, Núñez and Pérez-Heras 98 ). This study also demonstrated an inverse association between vasodilation and cholesterol:HDL ratio, suggesting that the effect of walnuts may have been mediated in part through an improved lipid profile( Reference Ros, Núñez and Pérez-Heras 98 ). It is well established that hypercholesterolaemia impairs endothelial function, which can be reversed by aggressive cholesterol lowering( Reference Adams, Kinlay and Blake 105 ). However, this study only demonstrated moderate cholesterol lowering, indicating that other mechanisms may also play a role. Investigators suggested that phenolic compounds in walnuts may have counteracted the pro-oxidant effects of PUFA on LDL. Mediterranean diets supplemented with 20–50 g walnuts/d( Reference Esposito, Giugliano and Giugliano 47 ) and 80–100 g pistachios/d( Reference Sari, Baltaci and Bagci 59 ) improved endothelial function by 21 and 24 %, respectively. Also observed with pistachio consumption was an improvement in glucose levels, lipid parameters, oxidative status and some indices of inflammation that may underlie the improved endothelial function( Reference Sari, Baltaci and Bagci 59 ). A diet with ad libitum consumption (56 g/d) of walnuts improved endothelial function by 45 % (effect size 0·6) in participants with type 2 diabetes( Reference Ma, Njike and Millet 72 ). Consumption of walnuts and walnut oil supplemented with flax seed (to boost the ALA content of the diet) increased endothelial function by 34 %, but no change was observed with the walnut diet alone( Reference West, Holub and Kris-Etherton 104 ). Of the five studies using higher doses (56–100 g/d) of nuts, four demonstrated benefits on endothelial function, indicating that higher doses may be required to elicit benefits( Reference Sari, Baltaci and Bagci 59 , Reference Ma, Njike and Millet 72 , Reference Ros, Núñez and Pérez-Heras 98 , Reference West, Holub and Kris-Etherton 104 ).
Of the studies, four did not show significant effects on flow-mediated dilatation. A hazelnut-enriched diet consumed by healthy men improved lipid parameters. In spite of this, endothelial functional improvement did not reach statistical significance( Reference Mercanligil, Arslan and Alasalvar 74 ). A quantity of 10–30 g nuts per d consumed by participants with either CVD risk or the metabolic syndrome also demonstrated no benefits on endothelial function( Reference Thomazella, Góes and Andrade 48 , Reference López-Uriarte, Nogués and Saez 106 ). Consumption of two doses (10 and 20 % of energy) of pistachios did not lead to a reduction in endothelial function( Reference West, Gebauer and Kay 107 ) despite relatively high doses of up to 126 g/d consumed for 4 weeks. The authors suggested that pistachios used were roasted which may have reduced polyphenol activity unlike walnuts used in other studies, which were not roasted before consumption.
Calculations of the weighted mean changes from nine of the ten studies indicated a 19·7 (95 % CI 4·3, 35·0) % relative increase in vasodilatation with nut consumption (Table 9). The effects of nuts on endothelial function demonstrate potential benefits, particularly walnuts. However, limited studies have been conducted with other types of nuts that may also demonstrate benefits. Endothelial dysfunction is often detected before increased blood pressure is observed and may be a more sensitive indicator than arterial compliance of early decline in vascular health; hence it may be a better target than blood pressure control or arterial compliance( Reference Ghiadoni, Taddei and Virdis 108 ).
Effects of nut consumption on arterial compliance
Studies measuring the effect of nut consumption on arterial compliance are found in Table 7. These include one cross-sectional study and one intervention study. A dose of 15 g walnuts/d consumed for 4 weeks demonstrated no effect on arterial stiffness( Reference Din, Aftab and Jubb 109 ). Whilst this dose is small, investigators chose a realistic amount likely to be consumed in free-living individuals for an extended period of time rather than higher doses used in other nut intervention studies. The cross-sectional study measured arterial compliance and compared quintiles of a healthy dietary pattern including nuts( Reference Nettleton, Schulze and Jiang 52 ). No association was found between a healthier diet pattern with an undetermined quantity of nuts and measures of arterial compliance. Few studies have investigated the effects of nuts on arterial compliance; therefore more studies in this area are warranted.
Effects of nut consumption on cognitive performance
There is little known of the impact of nut consumption on cognitive function. Studies measuring the effect of nut consumption on cognitive performance are found in Table 8. A 5-year prospective cohort study demonstrated a positive association between nut consumption and cognitive performance, equivalent to a substantial age reduction effect of 5–8 years in the highest-nut consumers (amount of nuts not specified)( Reference Nooyens, Bueno-de-Mesquita and van Boxtel 110 ). In addition, cognitive performance did not decline over the 5-year period in the highest-nut consumers. In a cross-sectional study (PREDIMED) an association was found between walnut consumption (but not other nuts) and improvements in performance on tests of working memory (see Table 8). In older adults nut consumption was associated with improved but non-significant scores for executive function in a cross-sectional study( Reference Nurk, Refsum and Drevon 111 ), with a low mean intake of nuts of 5 g/d. Only one intervention study in human subjects has been performed; this was conducted with students consuming 60 g ground walnuts/d for 8 weeks( Reference Pribis, Bailey and Russell 112 ). The study demonstrated a medium effect size (0·4) for improvement in inferential reasoning; however, other cognitive tests demonstrated no change. Despite the lack of intervention trials, observational studies indicate that long-term consumption of even small amounts of nuts may elicit benefits for cognitive function and reduction in cognitive decline. More evidence is needed from controlled intervention studies before a conclusive benefit can be determined.
Proposed mechanisms
Several nutrients in nuts may be responsible for observed improvements in cardiometabolic and cognitive measures. Tree and ground nuts have similar nutrient profiles, with some variations in micro- and macronutrients. From the studies reviewed (with the exception of walnuts, which have been more extensively researched than other nuts), it is not possible to determine differences in efficacy between different types of tree and ground nuts. Walnuts differ from other nuts in their greater antioxidant capacity, polyphenol and ALA content (see Table 1). ALA found in walnuts is associated with improved endothelial function( Reference Mozaffarian 31 ), inflammation( Reference Winnik, Matter and Lohmann 113 ) and neuroprotection in animal models( Reference Stark, Crawford and Reifen 114 ) and is hypothesised to maintain cognitive function in older adults( Reference Freemantle, Vandal and Tremblay-Mercier 115 ). Other unsaturated fatty acids in nuts may be beneficial for insulin sensitivity( Reference Risérus 116 ) and evidence suggests that higher intakes are associated with a lower risk of type 2 diabetes( Reference Salmerón, Hu and Manson 117 ), whereas higher intakes of SFA adversely affect glucose metabolism and insulin resistance( Reference Vessby, Uusitupa and Hermansen 118 – Reference Ebbesson, Tejero and López-Alvarenga 120 ). There is also recent evidence to indicate that MUFA may contribute to improvements in arterial stiffness as well as endothelial function and inflammation( Reference Fuentes, López-Miranda and Sánchez 121 – Reference Bellido, López-Miranda and Pérez-Martínez 124 ). Consumption of a Mediterranean diet that is also high in MUFA has been shown to reduce VCAM-1 and E-selectin gene expression by almost half. Animal and human studies have demonstrated that inflammation can be modified by the intake of l-arginine( Reference Heffernan, Patvardhan and Ranadive 125 ). Individuals with hypercholesterolaemia have impaired synthesis of NO; supplementation of 7 g l-arginine/d in this population group has demonstrated benefits( Reference Clarkson, Deanfield and Adams 126 ), increasing endothelial-dependent dilatation by almost 3·5-fold. Nuts contain approximately 2–3 g arginine/100 g; hence doses of 30 g/d or more used in most studies could partly account for the improvement in endothelial function observed. Nuts also contain fibre and, when consumed with their skin intact, contain a significant amount of polyphenols( Reference Bolling, Chen and McKay 35 , Reference Sanders, McMichael and Hendrix 127 ), which have previously been shown to target endothelial cells resulting in improved vascular function( Reference Brock, Davis and Irving 42 , Reference Ghosh 128 , Reference Wong, Buckley and Coates 129 ). Fibre intake can also increase insulin sensitivity( Reference Bodinham, Smith and Wright 130 , Reference Weickert, Mohlig and Schofl 131 ). Vitamin E found in nuts may have a role in modifying some of the inflammatory mediators and may be beneficial for cognitive performance( Reference Singh, Devaraj and Jialal 38 , Reference Joshi and Praticò 40 ). γ-Tocopherol is a powerful antioxidant abundant in walnuts, Brazil nuts and pistachios; however, its effect on markers of cardiovascular risk including endothelial function and inflammation has not yet been determined. Nuts are naturally rich in K and Mg, which may facilitate blood pressure reductions unless consumed in the salted form( Reference Sacks, Svetkey and Vollmer 132 ). In addition, Mg, which has been inversely related to serum CRP levels, has the potential to improve inflammation in individuals with low Mg status( Reference Nielsen 133 ) and Mg intake is inversely associated with a reduced risk of type 2 diabetes( Reference Dong, Xun and He 134 ).
There is emerging evidence that frequent nut consumption beneficially affects cardiovascular risk beyond cholesterol lowering. Key mechanisms include anti-inflammatory, antioxidant and endothelial function, reduction in body fat and improvement in glucose metabolism, which play a central role in the development of atherosclerosis( Reference Landmesser, Hornig and Drexler 135 , Reference de Lorgeril, Boucher and de Leiris 136 ). Endothelial function is essential for cerebral vascular function to provide adequate cerebral blood flow to deliver nutrients (primarily glucose and oxygen) to the brain. It has been hypothesised that by improving blood-flow regulation in the brain, cognitive performance is also improved( Reference Sinn and Howe 6 , Reference Krestin, van der Lugt and Poels 7 ). Nutritional interventions that have demonstrated improvements in cerebral blood flow include n-3 fatty acids in fish oil( Reference Jackson, Reay and Scholey 137 ), polyphenols in cocoa( Reference Fisher, Sorond and Hollenberg 138 ) and wild green oats( Reference Wong, Berry and Buckley 139 ). Anti-inflammatory medications offer some protection from Alzheimer's disease, which is consistent with the hypothesis that damage to brain cells is part of an overall inflammatory reaction. If inflammation is the key, then nuts which contain anti-inflammatory nutrients, such as polyphenols, vitamin E and n-3 fatty acids may prove to be important to reduce damage to the brain.
Conclusions
The results summarised in the present study provide evidence that regular nut consumption may have a protective effect on both vascular health and cognition. These benefits were evident in trials with doses of higher intakes (>30 g/d) for extended periods (several weeks or longer). These findings further support the use of nuts to reduce cardiometabolic dysfunction and highlight their potential to maintain or restore endothelial function. This in turn could improve cerebral blood flow and hence cognitive performance as illustrated in Fig. 1. No published studies to date have measured the effect of nut consumption on cerebral blood flow and few studies have measured the impact of nuts on arterial compliance and cognitive performance. Whilst intervention studies have investigated the impact of nuts on endothelial function, only one study has taken the next step and considered whether nuts may have beneficial effects on cognitive performance. Further clinical studies are warranted to determine the type and dose of nut and duration of consumption and which populations may benefit.
Acknowledgements
J. A. B. is funded by a scholarship from the Australian Research Council linkage grant in partnership with the Peanut Company of Australia (no. LP100200597).
There are no declarations of conflict of interest.














