Introduction
P plays an essential role in agriculture and the food industry as a mineral present in ingredients used for livestock feeding as well as in mineral fertilisers, with about 90 % of the world's mined phosphate rock being used in the agriculture and food sector(Reference Smil1). According to recent estimates, global phosphate resources may be exhausted within the next 50–100 years(Reference Steen2, Reference Cordell, Drangert and White3). Thus, a sustainable resource management and a reduction of phosphate mining are becoming particularly important(Reference Rodehutscord4). In feedstuffs of plant origin, P is either present as non-phytate-P or as phytate-P, i.e. P bound to phytic acid (myo-inositol-1,2,3,4,5,6-hexakisphosphate; InsP6). Phytate is the main storage form of P, and can be found in seeds, plant tissues and plant organs(Reference Eeckhout and De Paepe5, Reference Steiner, Mosenthin and Zimmermann6). Plant seeds, which are the major feed components in diets for pigs, contain about 75 % of phytate(Reference Raboy7). In non-ruminant animals, hydrolysis of phytate is incomplete, as the proximal gastrointestinal tract (GIT) lacks sufficient enzymes such as endogenous mucosal phytase and phosphatase(Reference Maenz and Classen8–Reference Onyango and Adeola10). As a consequence, non-hydrolysed phytate is excreted with manure, possibly leading to an accumulation of P in the soil and water(Reference Whalen and Chang11, Reference Adeli, Sistani and Rowe12). Furthermore, phytic acid is known to act as an anti-nutritive agent, reducing the absorption of trace elements and minerals(Reference Schlemmer, Jany and Berk13), and interfering with digestion and absorption of dietary proteins and carbohydrates(Reference Selle, Cowieson and Cowieson9, Reference Rutherfurd, Chung and Moughan14, Reference Ravindran, Cabahug and Ravindran15). P is an essential constituent of many important primary cell metabolites such as lipopolysaccharides, phospholipids, nucleic acids and different cytoplasmic solutes(Reference Abelson16, Reference Hirota, Kuroda and Kato17). Several studies with various species suggest that P performs important functions within the immune system, with modifications in dietary P supply being related to modulations in immune function(Reference Kegley, Spears and Auman18–Reference Liu, Ru and Cowieson20). Kegley et al. (Reference Kegley, Spears and Auman18), for example, observed in weaned piglets that an increasing concentration of available P in the diet enhanced the cell-mediated immune response, but reduced the humoral immune response. According to in vitro studies with human and mammalian cells, specific lower phosphorylated inositol phosphates (InsPs) (for example, myo-inositol triphosphates (InsP3) and myo-inositol tetraphosphates (InsP4)) also play a significant part in cellular signal transduction, regulation of cell function, growth and differentiation after absorption(Reference Sauer and Cooke21). InsP4 is believed to be involved in T and B cell development and in neutrophil and mast cell functions(Reference Miller, Chamberlain and Cooke22). However, P is not only essential for the host animal, but also for the microbiota colonising the animal's GIT. It is part of important basic modules such as phospholipids in the cytoplasmic and outer membranes of Gram-negative bacteria and co-enzymes, as well as teichoic acid and nucleotides in the cell walls of Gram-positive bacteria(Reference Lengeler, Drews and Schlegel23, Reference Durand and Komisarczuk24). The potential impact of P on the intestinal microbiota is not only restricted to members of the indigenous microbiota, but may also apply to potentially pathogenic bacteria. There is evidence, for example, that the host's InsP metabolism is closely associated with specific intestinal pathogenic bacteria that have developed complex strategies to modulate their uptake and intracellular lifestyle(Reference Hilbi25, Reference Weber, Ragaz and Hilbi26). In both humans and animals, a stable endogenous microbiota is involved in colonisation resistance against incoming pathogens. Any change in the intestinal microbial ecosystem could therefore shift the balance between protective microbiota and pathogens in favour of the pathogens(Reference Stecher, Robbiani and Walker27, Reference Bearson, Allen and Bearson28).
Apart from pathogen pressure, the intestinal microbial equilibrium is influenced by a wide range of other factors, including host genetics, age, stress, the environment and diet(Reference Kiarie, Romero and Nyachoti29–Reference Mariat, Firmesse and Levenez33). Modulation of dietary protein level, carbohydrate composition and use of feed additives such as pre- and probiotics are among the dietary strategies currently implemented to support a healthy GIT microbiota in pigs(Reference Bauer, Williams and Smidt34). Moreover, several studies in pigs indicate an impact of dietary P and Ca on microbiota composition and bacterial activity in the GIT(Reference Metzler-Zebeli, Zijlstra and Mosenthin35, Reference Metzler-Zebeli, Mann and Schmitz-Esser36). The present review focuses on the impact of dietary P, phytate and phytate hydrolysis products on the porcine immune system and on the porcine microbiota along the GIT. Possible interactions between diet composition, the intestinal microbial equilibrium and the host's defence mechanisms, including a potential impact on host health, will be discussed. The importance of this aspect for both the host and its inhabiting microbiota will also be assessed. While the main focus of the present review is directed to the pig, studies on the effect of P supply on the porcine immune system are rare. Thus, studies on other species are also included when applicable.
The porcine immune system: a brief overview
The immune system protects the organism against pathogens and controls the integrity of the body. In vertebrates, it is composed of an innate and an adaptive arm, both of which include humoral and cellular components. The first line of defence is formed by innate immune cells, such as neutrophil granulocytes, natural killer cells, monocytes/macrophages, dendritic cells and endothelial cells, as well as humoral components such as the complement system. A more detailed overview of the porcine innate immune system has been given by Mair et al. (Reference Mair, Sedlak and Käser37). The characteristic cells of the adaptive immune response are T- and B-lymphocytes, with the antibodies released by B-lymphocytes forming the humoral part of the adaptive arm(Reference Shishido, Varahan and Yuan38). The main characteristic of the T and B cells is their specific recognition of antigens and the formation of an immunological memory, while cells of the innate immune system have the ability to distinguish between pathogens and self- or non-pathogenic structures. In this context, macrophages, dendritic cells and other cells of the innate arm express pattern recognition receptors such as Toll-like receptors and lectins(Reference Kumar, Kawai and Akira39). The binding of conserved pathogen structures (such as lipopolysaccharides of bacteria or unmethylated CpG of viral nucleic acid by the Toll-like receptors) induces cell activation and the release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. The T and B cells recognise antigens through their T cell receptor (TCR) and B cell receptor, respectively. The B cell receptor is a surface-bound form of an antibody and can recognise native proteins. The TCR is composed of two protein chains, either αβ or γδ, and the expressing cells are either named αβ-T cells or γδ-T cells. In contrast to TCR γδ-T cells, TCR αβ-T cells recognise small peptide fragments only if bound to the cell-recognition molecule major histocompatibility complex (MHC). The TCR αβ-T cells can be further differentiated into cells expressing either the TCR co-receptor CD4 or CD8. The CD4+T cells recognise antigens presented on MHC class II molecules, while CD8+T cell subsets recognise antigens presented on MHC class I molecules(Reference Charerntantanakul and Roth40). Upon binding to an antigen, B and T cells differentiate from a mature, but naive status into potent effector cells comprising various activities such as killing, cytokine production, or antibody production. After clearing an infection, some of these differentiated cells survive and sustain in the body, now able to react promptly to the same pathogen.
The GIT represents one of the largest immunological organs of the body. For the mucosal immune system consisting of innate and adaptive arms, it is important to achieve a balance between immune tolerance and immune responsiveness in a diverse milieu of harmful pathogens, dietary antigens and the intestinal microbiota harbouring the GIT(Reference Artis41). Vertebrates have specialised lymphoid structures, such as the gut-associated lymphoid tissue (GALT), which provides a specific host defence and constitutes the largest accumulation of immunological cells in the organism(Reference Mowat and Viney42). The GALT is divided into inductive sites, including Peyer's patches, and into effectors sites(Reference Brandtzaeg and Pabst43), such as the lamina propria (Reference Nagler-Anderson44). For pigs in particular, the various components of the GALT need to sustain intestinal homeostasis during the critical period from birth to weaning, in order to ensure optimal growth and health conditions(Reference Burkey, Skjolaas and Minton45). For a comprehensive overview of the GIT immunity and of the components of the mucosal immune system with special focus on the pig, see Burkey et al. (Reference Burkey, Skjolaas and Minton45).
The intestinal microbiota: composition and interaction with the immune system of the host
The intestinal microbial ecosystem is characterised by a high density of different species in a very complex and dynamic environment. The amount of microbial cells in single-stomached animals, including humans, exceeds the amount of host cells by at least one order of magnitude(Reference Savage46). The GIT of single-stomached animals harbours all groups of microbes, but mainly bacteria(Reference Gaskins, Lewis and Southern31, Reference Savage46). It is colonised by Gram-positive bacteria including Streptococcus (aerotolerant), Lactobacillus, Bifidobacterium (microaerobe or obligate anaerobe), Clostridium (obligate anaerobe), but also by obligate anaerobe Gram-negative bacteria such as Bacteroides, Fusobacterium, Selenomonas and Prevotella (Reference Gaskins, Lewis and Southern31). Firmicutes and the Bacteroidetes phyla form the major group of the gut microbiota(Reference Leser, Amenuvor and Jensen47, Reference Kim, Borewicz and White48). The bacterial density increases from the proximal to the distal GIT. About 500 bacterial species and 10Reference Onyango and Adeola10–1011 bacteria per g digesta colonise the porcine colon and caecum(Reference Gaskins, Lewis and Southern31, Reference Jensen, Piva, Bach Knudsen and Lindberg49, Reference Rist, Weiss and Eklund50).
The intestinal microbiota has coevolved over millions of years, so that the relationship between the indigenous microbes and the host may be more intensive than previously assumed. These findings suggest that ecological development is influenced by the genetics of the host and by the interaction with the coevolved intestinal microbiota(Reference Darveau, McFall-Ngai and Ruby51, Reference Hooper52). Apart from host genetics, the composition of the microbiota in the GIT, for example of humans, pigs and poultry, is affected by several factors. These include pathogen pressure in particular, but also age, environment, stress, diet composition (for example, type and content of carbohydrates, lipids, proteins and minerals)(Reference Kiarie, Romero and Nyachoti29–Reference Mariat, Firmesse and Levenez33), and endogenous nutrient sources(Reference Stewart, Hillman, Maxwell, Gansworthy and Cole30). Moreover, intestinal pH, inhibitory factors such as antimicrobial substances produced by members of the microbial ecosystem, fermentation endproducts and digesta flow rate may also affect microbial composition and activity(Reference Gaskins, Lewis and Southern31, Reference Pluske, Pethick and Hopwood32). The main energy source of the intestinal microbiota is fermentable carbohydrates passing through the GIT, such as non-digestible oligosaccharides, NSP and different types of resistant starch(Reference Houdijk, Hartemink and Verstegen53). The saccharolytic activity of the microbes is associated with positive effects for the host(Reference Geboes, De Hertogh and De Preter54) due to the production of SCFA including butyrate, acetate and propionate(Reference Varel and Yen55). These SCFA serve as energy sources for the host, and can meet about 30 % of the energy needs of a growing pig(Reference Morita, Kasaoka and Kiriyama56). Butyrate represents the main energy source for colonic epithelial cells(Reference Pryde, Duncan and Hold57), and is commonly produced by clostridia such as Roseburia species or Eubacterium rectale (Reference Aminov, Walker and Duncan58). In contrast to butyrate and propionate, which are produced by a small number of bacterial groups, acetate is formed by many different bacterial species(Reference Cummings and Englyst59). On the other hand, the fermentation of dietary proteins results in the production of detrimental substances such as amines, phenols and ammonia(Reference Jensen, Piva, Bach Knudsen and Lindberg49), and is often associated with the growth of potentially pathogenic microbes such as Bacteroides and Clostridium species(Reference Macfarlane, Macfarlane, Gibson and Macfarlane60).
The microbial composition is affected by different immunological mechanisms including antibacterial proteins of the innate immune response such as α-defensins. Paneth cells of the small intestine produce these peptides, which may change the microbial composition as it has been shown for mice(Reference Salzman, Ghosh and Huttner61). The intestinal immune system controls the bacterial exposure to the tissue of the host, thereby reducing the risk of infection. Two main immunological mechanisms exist to prevent the host from colonisation with intestinal pathogens, including the reduction of direct contact with the epithelia cell surface (stratification), and the restriction of the penetration of immunological compartments (compartmentalisation)(Reference Hooper, Littman and Macpherson62). The stratification of intestinal microbiota, particularly bacteria, on the luminal tissue is regulated by secreted IgA which prevents microbial translocation across the epithelial barrier(Reference Macpherson and Uhr63, Reference Macpherson, Gatto and Sainsbury64). Mucosal compartmentalisation reduces the exposure of bacteria to the systemic immunological compartments, for example by cytokine-producing innate lymphoid cells of the lamina propria (Reference Spits and Di Santo65). Detailed descriptions of the interaction between the immune system and the microbiota have been published elsewhere(Reference Hooper, Littman and Macpherson62).
Dietary modulators affecting phosphorus digestibility and nutrient–phytate interactions
P contributes substantially to skeletal development, mineral metabolism, energy metabolism, cellular signalling and the stabilisation of cell membranes(Reference Takeda, Yamamoto and Nashiki66, Reference Taylor and Bushinsky67). In pigs, the small intestine, especially the jejunum, has been suggested as the major site of P absorption(Reference Breves and Schröder68). On the other hand, results concerning the role of the large intestine in the regulation of P absorption are rather contradictory. Both absorption(Reference Den Hartog, Huisman and Thielen69–Reference Seynaeve, Janssens and Hesta71) and secretion of P(Reference Larsen and Sandström72) are supposed to occur in the large intestine of pigs. Moreover, it has been suggested by several authors that factors such as dietary P and Ca level(Reference Jongbloed, Mroz and Kemme73–Reference Ruan, Zhang and Yin75), phytate content(Reference Schlemmer, Jany and Berk13, Reference Jongbloed, Mroz and Kemme73), ingredient composition of the diet(Reference Fang, Li and Yin76), feeding level and supply of inorganic P sources(Reference Rodehutscord, Faust and Pfeffer77) may influence P homeostasis across the GIT.
Phytate hydrolysis
In ruminants, phytate is largely degraded due to the activity of rumen microbiota, including several anaerobic phytase-producing bacteria such as Selenomonas ruminantium, Megasphaera elsdenii, Prevotella ruminicola and Mitsuokella multiacidus (Reference Yanke, Bae and Selinger78), thus rendering P available for the host. In contrast, only a partial hydrolysis of phytate occurs in non-ruminants, which lack sufficient hydrolytic enzymes such as endogenous phytase in the proximal GIT to hydrolyse dietary phytate. Recent studies with broilers fed diets low in P and Ca have shown, however, that about two-thirds of dietary InsP6 can be degraded in the GIT(Reference Shastak, Zeller and Witzig79), indicating substantial activity of enzymes originating from a mix of endogenous and microbial sources. Another reason for the limited degradation of phytate is the insolubility of mineral Ca–phytate complexes at the pH levels usually prevalent in the small intestine(Reference Selle, Cowieson and Ravindran80). As a consequence, the P digestibility is reduced due to the formation of insoluble Ca–phytate complexes and their negative effect on the efficacy of mucosal phytase(Reference Steiner, Mosenthin and Zimmermann6). Consequently, there is a need for dietary supplementation with inorganic phosphate and/or exogenous phytases to increase the animals’ efficiency of P utilisation.
Phytase activities
Exogenous phytases of microbial origin are extensively used as feed additives in non-ruminant diets to improve digestibility of P in plant feed ingredients. Due to the acidic conditions of the stomach, phytate is relatively soluble and can be hydrolysed by supplemental exogenous phytases(Reference Selle, Cowieson and Cowieson9). Varying amounts of InsP6-P can also be utilised by the animal due to the presence of intrinsic phytases in some feed ingredients(Reference Eeckhout and De Paepe5, Reference Düngelhoef, Rodehutscord and Spiekers81). As a result, hydrolysis of InsP6-P to inorganic P and lower myo-inositol phosphates such as myo-inositol pentaphosphates (InsP5), InsP4 and InsP3 occurs(Reference Ariza, Moroz and Blagova82). However, phytase activity varies considerably between different plant feedstuffs(Reference Steiner, Mosenthin and Zimmermann6). Cereals and cereal by-products, for example, have a higher intrinsic phytase activity compared with legume seeds such as soyabeans and field beans(Reference Eeckhout and De Paepe5, Reference Viveros, Centeno and Brenes83, Reference Zimmermann, Lantzsch and Langbein84). In a recent study by Metzler-Zebeli et al. (Reference Metzler-Zebeli, Deckardt and Schollenberger85), the treatment of barley with lactic acid alone or in combination with heat treatment also resulted in a reduced InsP6-P concentration, possibly due to a decrease in pH. At the same time, the formation of lower phosphorylated phosphates such as InsP4 and InsP5 was stimulated. According to the authors, further studies are required to confirm these effects on intestinal P digestibility and to elucidate the impact on animal performance(Reference Metzler-Zebeli, Deckardt and Schollenberger85). According to Blaabjerg et al. (Reference Blaabjerg, Nørgaard and Poulsen86–Reference Blaabjerg, Carlson and Hansen-Møller88), heat treatment affects phytate degradation in diets and feedstuffs for pigs in combination with phytase supplementation, both in vitro and in vivo. In one in vitro study(Reference Blaabjerg, Carlson and Hansen-Møller88), phytate degradation was enhanced upon addition of phytase to heat-treated diets compared with non-heat-treated diets. The authors suggest that structural changes induced by the heat treatment might have enhanced the contact between phytase and phytate, leading to increased phytate degradation.
Carbohydrate–phytate interactions
Several authors(Reference Jongbloed, Mroz and Kemme73, Reference Partridge89) have suggested interactions between carbohydrate composition of the diet and P disappearance across the gut wall, especially in the large intestine. In growing pigs fitted with simple T-cannulas, for example, the addition of different fermentable carbohydrates at a level of 25 % to a diet containing low contents of digestible P present as phytate-P differently affected the net disappearance of P in the large intestine(Reference Baumgärtel, Metzler and Mosenthin90). In this study, cellulose and pectin caused a net secretion of P into the lumen of the large intestine, whereas supplemental starch led to a net absorption of P. Partridge(Reference Partridge89) found a significant net secretion of P into the large intestine when pigs were fed maize starch sucrose and groundnut meal, but not for a cereal-based diet. Jongbloed et al. (Reference Jongbloed, Mroz and Kemme73) also observed a net secretion into the large intestine for a maize–soyabean meal-based diet, and, by contrast, a net absorption from the large intestine for a diet based on tapioca and hominy feed. It appears that the intestinal net disappearance of P is affected by the source of fermentable carbohydrates in pigs’ diets. According to Baumgärtel et al. (Reference Baumgärtel, Metzler and Mosenthin90), a stimulation of microbial growth as a consequence of an increased supply with fermentable carbohydrates (for example, pectin) would be associated with the increased incorporation of P into microbial biomass, thus increasing the demand for P. As a result, a stimulation of P secretion might occur. Furthermore, the carbohydrate sources used in these studies could have affected the composition and metabolic activity of the intestinal microbiota, thereby also influencing microbial degradation of InsP6. Baumgärtel et al. (Reference Baumgärtel, Metzler and Mosenthin90) observed in their study a reduced ileal InsP6 degradation for starch. The authors suggested that proliferation of lactic acid bacteria due to the presence of high amounts of starch at the terminal ileum might have suppressed other bacteria with a higher potential for phytase production. Although further research on the influence of fermentable carbohydrates on intestinal net disappearance of P is required, results of different studies suggest a close relationship between the composition and metabolic activity of the intestinal microbiota and P homeostasis across the gut wall, thus emphasising the importance of dietary components being capable of modulating the intestinal microbiota.
Effects of phosphorus on the immune system
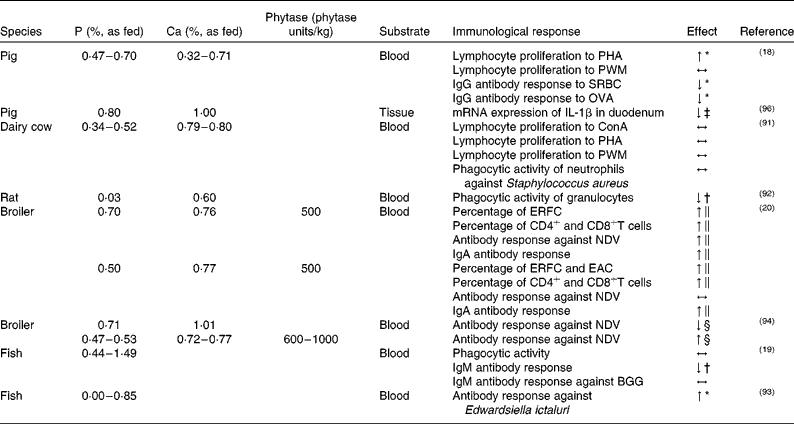
As illustrated in Table 1, few studies have so far examined the impact of dietary P on immune cell functions such as lymphocyte proliferation(Reference Kegley, Spears and Auman18, Reference Mullarky, Wark and Dickenson91), phagocytic activity(Reference Jokinen, Vielma and Aaltonen19, Reference Mullarky, Wark and Dickenson91, Reference Kiersztejn, Chervu and Smogorzewski92), antibody response(Reference Kegley, Spears and Auman18–Reference Liu, Ru and Cowieson20, Reference Eya and Lovell93, Reference Ghahri, Rostami and Zandiyeh94), numbers of leucocytes(Reference Liu, Ru and Cowieson20) and further parameters(Reference Zyla, Wikiera and Koreleski95, Reference Metzler-Zebeli, Gänzle and Mosenthin96) in blood or tissues related to the immune system.
Table 1 Effect of dietary phosphorus–calcium level and phytase supplementation on immune parameters measured in blood or tissue of different species

PHA, phytohaemagglutinin; ↑ , higher; PWM, pokeweed mitogen; ↔ , no difference; SRBC, sheep erythrocytes; ↓ , lower; OVA, ovalbumin; ConA, concanavalin A; ERFC, erythrocyte rosette-formation cells; NDV, Newcastle disease virus; EAC, erythrocyte antibody complement cells; BGG, bovine γ-globulin.
* With increasing dietary P content.
† Compared with high-CaP diet.
‡ Compared with low-CaP diet.
§ Compared with diet according to the actual P requirement.
∥ Compared with diet without phytase supplementation.
Lymphocyte proliferation
It appears that either through dietary supplementation with P(Reference Kegley, Spears and Auman18) or increased P availability upon phytase addition(Reference Liu, Ru and Cowieson20) the proliferation and function of peripheral lymphocytes in different species can be stimulated, thereby supporting the adaptive arm of the immune system. However, studies in pigs are rare. There is only one study by Kegley et al. (Reference Kegley, Spears and Auman18) with weaned pigs in which the effect of different dietary P levels on mitogen-induced lymphocyte proliferation has been examined so far. The results indicate a positive linear relationship between phytohaemagglutinin-induced lymphocyte proliferation and the amount of dietary P. While phytohaemagglutinin primarily stimulated T lymphocytes, no effect of lymphocyte proliferation on pokeweed mitogen could be observed, which suggests that B cell proliferation is not affected. According to the authors, reduced T cell proliferation might be due to the circulation of vitamin D in the organism. This finding is supported by the results of a study by Engstrom et al. (Reference Engstrom, Horst and Reinhardt97), in which a low-P diet enhances the concentration of plasma 1,25-dihydroxyvitamin D3, a vitamin known to inhibit several lymphocyte functions including the proliferation response to mitogen stimulation(Reference Lemire, Adams and Sakai98, Reference Tsoukas, Provvedini and Manolagas99). As a consequence, feeding pigs below their actual P requirement(100) impairs lymphocyte function, although different lymphocyte subsets seem to be differently affected. Moreover, the impact of low-P diets on lymphocyte function might not be the same for all species as microbial phytase production and release in the gut might have modulating effects on lymphocytes. In lactating dairy cows, for example, Mullarky et al. (Reference Mullarky, Wark and Dickenson91) failed to observe an effect of differences in P supply on lymphocyte proliferation in response to the mitogens concanavalin A (ConA), pokeweed mitogen and phytohaemagglutinin.
Activity of phagocytes
The effect of P on the phagocytic function of immune cells has so far only been studied in rats, ruminants and European whitefish (Coregonus lavaretus L.). In a study by Kiersztejn et al. (Reference Kiersztejn, Chervu and Smogorzewski92) the impact of differences in dietary P supply on the phagocytic activity of granulocytes in rats was assessed. The animals received either a low- or a high-P diet with or without injection of a Ca antagonist (verapamil), with verapamil often administered to prevent a rise of Ca2+ levels in the blood. The phagocytic activity of granulocytes was significantly lower in rats fed the low-P diets compared with high-P diets, irrespective of application of the Ca antagonist. Furthermore, a higher Ca2+ level and lower ATP content in granulocytes were observed in rats fed the low-P diet without verapamil. The low-P diet together with verapamil yielded lower ATP contents in granulocytes as compared with rats fed the high-P diets with or without verapamil treatment. The authors concluded that dietary phosphate depletion increased cytosolic Ca2+ levels and decreased ATP concentration of granulocytes, which, in turn, resulted in an impaired phagocytic activity(Reference Kiersztejn, Chervu and Smogorzewski92). Similarly, Massry(Reference Massry101) observed elevated cytosolic Ca2+ levels and an impaired phagocytosis of granulocytes in rats fed a low-P diet.
In a study with dairy cows, Mullarky et al. (Reference Mullarky, Wark and Dickenson91) found no effect of dietary P supply on the bactericidal activity of neutrophils against Staphylococcus aureus. The authors assumed that even the lowest dietary P concentration used in their study was still too high to exhibit adverse effects on ATP levels and phagocytic activity, which is in contrast to findings in rats(Reference Kiersztejn, Chervu and Smogorzewski92). A study conducted with juvenile European whitefish(Reference Jokinen, Vielma and Aaltonen19) also failed to demonstrate an effect of dietary P supplementation on phagocytic activity.
It can be concluded from the results of several studies(Reference Jokinen, Vielma and Aaltonen19, Reference Mullarky, Wark and Dickenson91, Reference Kiersztejn, Chervu and Smogorzewski92) on the functional activity of immune cells that a sufficiently high P availability is essential to maintain normal immune cell functions. There is also sufficient evidence for the existence of species-specific differences, such as effects of a low dietary P supply are apparently more pronounced in non-ruminant mammals such as pigs than in ruminants, where more P is available to the host due to the activity of the rumen microbiota, including several phytase-producing bacteria(Reference Yanke, Bae and Selinger78).
Lymphocyte distribution
In addition to immune cell functions, adequate distribution of lymphocytes and antigen-presenting cells in the organism is generally critical for immune functioning. The effect of dietary P supply on the number and distribution of immune cells has yet not been studied in mammalian livestock. However, according to the results of a study by Liu et al. (Reference Liu, Ru and Cowieson20) with poultry, both dietary phytate content and phytase activity can affect blood T and B cell numbers. The authors determined the percentage of erythrocyte rosette-forming cells (ERFC, T cells) and erythrocyte antibody complement cells (EAC, B cells and monocytes), as well as the percentage of CD4+ and CD8+T cells by flow cytometry. The percentage of ERFC was higher in broilers that had received a high-phytate diet (0·44 %, as fed) supplemented with phytase (500 and 1000 phytase units/kg of feed) as compared with groups without phytase supplementation. Moreover, the percentages of ERFC and EAC were higher in broilers fed the low-phytate diets (0·22 %, as fed) supplemented with phytase, compared with the low-phytate diets without phytase supplementation. In addition, the percentage of blood CD4+ and CD8+T cells was elevated in broilers with phytase-supplemented diets. Obviously, the increased availability of P following phytase-mediated degradation of phytate into lower phosphorylated InsPs resulted in higher concentrations of ERFC, EAC and blood CD4+ and CD8+T cells. Similarly, in a study with mice(Reference Miller, Sandberg and Huang102) investigating possible interactions between InsP metabolism and specific immunological parameters, a modulation of B cell selection and activation due to Ins(1,3,4,5)P4 could be observed. In a study with growing broilers(Reference Zyla, Wikiera and Koreleski95), the effect of low-P diets supplemented with a phytase-containing ‘cocktail’ on the mass of immune organs and the area of intestinal surface covered by GALT was examined. Among other factors, the addition of the ‘cocktail’ containing phytase, acid phosphatase, pectinase and citric acid to the low-P control diet increased the mass of the bursa fabricius as compared with a low-P control diet without enzyme supplementation. The aforementioned studies(Reference Liu, Ru and Cowieson20, Reference Zyla, Wikiera and Koreleski95) with birds suggest that P content or P availability may influence lymphocyte migration.
Antibody response
The ability of organisms in establishing an appropriate antibody response depends on the timely meeting of immune-competent cells such as lymphocytes and antigen-presenting cells in lymphatic organs. The key processes – function and migration of immune cells – seem to be altered by insufficient dietary P supply as outlined above. Studies using experimental immunisation protocols showed both negative and positive consequences of the P supply. In a study with weaned pigs, Kegley et al. (Reference Kegley, Spears and Auman18) examined the effect of different dietary P supplies on the antibody response. Upon immunisation with either sheep erythrocytes or ovalbumin, IgG antibody response decreased with increasing P supply. The lowest concentration of IgG antibodies against sheep erythrocytes could be detected for the diet with the highest content of P. At first glance, this finding seems to challenge the observed effect of P content on lymphocyte proliferation. However, according to Kegley et al. (Reference Kegley, Spears and Auman18), an increased plasma level of 1,25-dihydroxyvitamin D3 in pigs fed a low-P diet might be beneficial for antibody production, as high levels of vitamin D3 are also known to stimulate monocytes, thereby enhancing antigen presentation(Reference Manolagas, Provvedini and Tsoukas103). In this case, the stimulatory effect of low-P content on monocytes would compensate for the negative effect on T cell function. However, this conclusion remains speculative, as vitamin D3 levels were not measured in this study.
In a study with fish(Reference Jokinen, Vielma and Aaltonen19), European whitefish were fed diets varying in P content. The authors observed lower total plasma IgM concentrations upon feeding of the low-P diet compared with the diet with the highest P content. In a subsequent experiment, the animals were fed the diet with the lowest and the highest P levels for 7 weeks, and were subsequently immunised with an intraperitoneal injection of bovine γ-globulin. As expected, the total plasma IgM concentration was lower in the fish fed the low-P diet compared with those fed the high-P diet, but no effect on bovine γ-globulin-specific IgM antibody response could be detected. In both experiments, the growth of fish that were fed the low-P diet was significantly lowered, however, possibly confounding the results as growth hormones are believed to affect immunological responses(Reference Murphy, Rui and Longo104). It has been shown, for example, that growth hormones have a stimulatory effect on human and murine T cells in vitro (Reference Snow, Feldbush and Oaks105, Reference Mercola, Cline and Golde106). Eya & Lovell(Reference Eya and Lovell93) assessed the effect of dietary P on the antibody response against Edwardsiella ictaluri in channel catfish (Ictalurus punctatus). In addition to the specific antibody production increase of E. ictaluri with increasing dietary P concentrations, mortality of the fish also decreased at higher dietary P levels. It appears that differences in dietary P content may affect total plasma Ig levels and the specific humoral antibody responses in fish.
Ghahri et al. (Reference Ghahri, Rostami and Zandiyeh94) studied the effect of different dietary P levels and microbial phytase supplementation on the antibody production in broilers vaccinated with the Hitcher B1 Newcastle disease virus (NDV). The anti-NDV antibody titre of broilers fed the low-P diet was lower in comparison with the group fed according to their actual P requirement. Moreover, adding phytase to the diet increased the antibody response against NDV. As phytase exerted a positive influence on the immune response to NDV, it was suggested that both phytase and a reduction in P supply may modulate immune functions. A study by Liu et al. (Reference Liu, Ru and Cowieson20) with broilers assessed the effect of phytate and phytase on serum anti-NDV antibodies and jejunal mucosal secretory IgA production after intranasal and intraocular administration of NDV attenuated vaccine. Jejunal secretory IgA production was increased by dietary phytase addition for the low- and high-phytate diets. For the high-phytate diets supplemented with phytase, the anti-NDV antibodies were enhanced, whereas adding phytase to the low-phytate diets showed no effect.
In conclusion, as influenced by the availability of dietary P, both total plasma Ig and antibody response are affected differently. However, the direction of this effect appears to be inconsistent across species. A dietary supplementation of phytate and phytase seems to positively affect the adaptive immune response, at least in fish and broilers, but not in pigs, except for phytase. This inconsistency across studies can be attributed to differences in the nature of the antigens, immunisation protocols and the dietary content of P and phytase. It also cannot be ruled out that stimulatory and inhibitory effects of P on different immune cell subsets exist, which probably compensate each other at different extents, depending on the source of antigen or species characteristics. In either case, the effect of P on the function and distribution of different immune parameters warrants further investigation.
Expression of intestinal cytokines
In weaned piglets, Metzler-Zebeli et al. (Reference Metzler-Zebeli, Gänzle and Mosenthin96) studied the influence of dietary oat β-glucan and CaP level on intestinal inflammation. The relative mRNA expression of IL-1β and IL-6 was analysed in tissue samples of the duodenum, the ileum, the caecum and the mid-colon. In the duodenum, the high-CaP diet decreased the expression of IL-1β compared with the low-CaP diet, irrespective of β-glucan supplementation. Since pro-inflammatory cytokines can affect intestinal permeability and nutrient transport directly and indirectly(Reference McKay and Baird107), it can be speculated whether the modulation of the intestinal expression of selected genes in pigs fed the high-CaP diet as observed by Metzler-Zebeli et al. (Reference Metzler-Zebeli, Gänzle and Mosenthin96) assists in maintaining intestinal function during the post-weaning period. Shaw et al. (Reference Shaw, van Ginkel and Macklin108) examined the effect of dietary phytase upon a natural Eimeria challenge in naive and vaccinated broilers. Broilers were fed maize–soyabean meal-based diets with one of two dietary CaP levels (low and according to their actual P requirement). At day 18, the expression of IL-17 was increased both in the duodenum of the vaccinated and the challenged broilers fed the low-CaP diet supplemented with phytase. As IL-17 plays an important role in the immunological control of infectious diseases and is a key factor in T-helper cell 17 lineage immune response(Reference Zhang, Liu and Song109), the observed increase in IL-17 expression suggests a health-promoting effect on the animal.
Conclusion and remarks on future research on phosphorus and immune function
The overall picture emerging from the current studies on P availability and immune functioning indicates a minimum requirement of P to ensure normal immune function. The critical amount of dietary P to maintain immunity, however, remains unclear and may substantially vary across species, for example between non-ruminants and ruminants due to their different access to phytate. Since researchers have observed contradictory effects of high P levels on different aspects of immunity, such as enhanced T cell proliferation but a diminished antibody response in pigs, future work should involve a comprehensive analysis of the different immune subsystems. It is also evident that animal growth may decrease upon feeding of low-P diets, at least in some studies and species(Reference Murphy, Rui and Longo104). This suggests that growth-related hormones with immune-modulatory properties such as somatotropin or cortisol have a direct impact on immune cell function and migration(Reference Webster Marketon and Glaser110, Reference Welniak, Sun and Murphy111). A properly functioning immune system, however, is crucial for organisms to resist infections and to prevent diseases. Current studies strongly suggest that this ability is dependent on dietary P levels, but further research is required to verify this hypothesis.
Effects of phosphorus on the intestinal microbiota
P contributes considerably to bacterial structure and metabolic processes(Reference Lengeler, Drews and Schlegel23, Reference Durand and Komisarczuk24). According to Wood & Clark(Reference Wood and Clark112), a surplus of P can be stored as polyphosphates in bacterial cells and may be used as an energy and P source for metabolic processes. Furthermore, P is important for bacterial proliferation. According to the results of in vitro studies, for example, the phosphate concentration of the culture medium has been shown to be positively correlated with the growth yield of Bacteroides amylophilus, an amylolytic and pectinolytic rumen bacterium(Reference Caldwell, Keeney and Barton113). Moreover, several authors have concluded from the results of in vitro studies(Reference Francis, Gawthorne and Storer114–Reference Metzler and Mosenthin116) that P as a coenzyme is essential for bacterial degradation of dietary fibre, and that bacterial synthesis of fibrolytic enzymes is strongly dependent on sufficient P supply. In an in vitro study of Francis et al. (Reference Francis, Gawthorne and Storer114), the effect of phosphate concentration on the bacterial cellulase activity of rumen fluid of fistulated merino sheep was assessed. Cellulases of rumen micro-organisms are extra-cellular enzymes; therefore they are exposed to stimulators or inhibitors present in the surrounding fluid. In this study, cellulase activity of the rumen fluid was progressively stimulated with rising phosphate concentrations from 5 to 50 mm. In another in vitro study using the rumen simulation technique, Komisarczuk et al. (Reference Komisarczuk, Merry and McAllan117) examined the effect of variations in P supply on rumen micro-organisms in sheep. P deficiency caused a reduction in SCFA synthesis due to reduced fermentation of cellulose. This suggests that the activity of bacterial fibrolytic enzymes is modulated by the content of available P in the surrounding medium(Reference Metzler and Mosenthin116). Metzler et al. (Reference Metzler, Mosenthin and Baumgärtel118) conducted a study with ileally cannulated growing pigs fed either a low-P maize–soyabean meal-based control diet, or 75 % of the control diet supplemented with either 25 % lignocellulose, maize starch or apple pectin in order to assess the impact of differently fermentable carbohydrates on P metabolism, on the chemical composition of faecal mixed bacterial mass (MBM), and on microbial activity in the large intestine. Upon addition of apple pectin or lignocellulose, P recoveries were higher in ileal than in faecal samples. Microbial activity (measured as SCFA production) was affected by the different fermentable carbohydrates used: for example, starch supplementation resulted in higher ileal SCFA contents when compared with supplementation with cellulose or pectin. Compared with the control, the P content of the MBM was decreased for the pectin treatment. The N:P ratio in the MBM was higher for the pectin diet, whereas the Ca:P ratio was constant for all treatments. The changes observed in the accumulation of P in the MBM as influenced by the differently fermentable carbohydrates may be related to a modulation of microbial activity, measured by differences in SCFA production. Pectin, for example, caused a reduction in P content of faecal MBM in comparison with the control diet, whereas ileal SCFA content was lower and faecal SCFA content was higher compared with the starch treatment. This would indicate a close relationship between the availability of fermentable substrate and P metabolism.
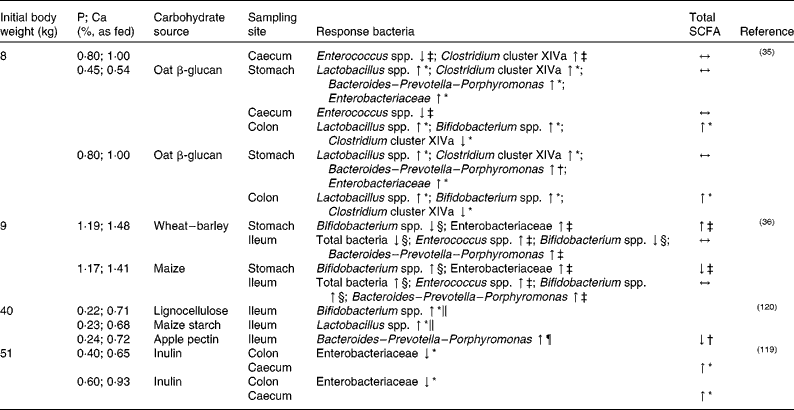
The impact of variations in dietary P supply and carbohydrate sources in the diet on the intestinal microbiota composition and activity in pigs has been reported in several in vivo studies(Reference Metzler-Zebeli, Zijlstra and Mosenthin35, Reference Metzler-Zebeli, Mann and Schmitz-Esser36, Reference Varley, McCarney and Callan119, Reference Metzler, Vahjen and Baumgärtel120) as summarised in Table 2. Metzler-Zebeli et al. (Reference Metzler-Zebeli, Mann and Schmitz-Esser36), for example, studied the effect of different cereal grains and variations in dietary CaP content on microbial composition and metabolites in weaned piglets. Piglets were fed for 14 d either a wheat–barley- or a maize-based diet with a CaP content according to their actual requirement or a high CaP content. Digesta samples of the stomach, ileum and mid-colon were analysed for specific bacterial groups using quantitative PCR. Generally, diet modulated bacterial composition and metabolites in the upper GIT, while no effects could be observed in the colon. In the stomach and in the ileum, a selective stimulation of Bifidobacterium could be observed upon feeding of the maize- v. the wheat–barley-based diet. In addition, higher numbers of some gastric and ileal bacterial groups such as gastric Enterobacteriaceae, ileal Enterococcus, Bacteroides–Prevotella–Porphyromonas and Campylobacter were observed when the diet with the high-CaP content was fed compared with the diet with a CaP content according to their actual requirement. The authors concluded that, although a selective stimulation of specific bacterial groups was observed in their study, the intestinal microbiota of weaned pigs is quite resistant to dietary modulations such as changes in carbohydrate and CaP content. We can conclude that further information on selective stimulation of certain beneficial bacterial groups, such as Bifidobacterium spp., will be useful for diet formulation in young pigs. On the other hand, given that Campylobacter species such as Campylobacter coli may cause diarrhoea(Reference Nic Fhogartaigh and Dance121) and are a source of highly immune potent lipopolysaccharides(Reference Mani, Weber and Baumgard122, Reference Zebeli and Metzler-Zebeli123), increased numbers of Campylobacter spp. are considered non-beneficial. Thus, attention should also be paid to possible harmful bacterial groups regarding gut health and growth performance(Reference Metzler-Zebeli, Mann and Schmitz-Esser36).
Table 2 Effect of dietary phosphorus and calcium level in combination with different carbohydrate sources on selected bacterial groups and total SCFA production in the intestine of weaned (initial body weight 8–9 kg) and growing (initial body weight 30–51 kg) pigs

↓ , Lower; ↑ , higher; ↔ , no difference.
* Compared with the control diet without carbohydrate supplementation.
† Compared each with the other.
‡ Compared with the diet with a CaP content according to the actual requirement/low-CaP diet.
§ Compared with the other cereal diet.
∥ Compared with the pectin treatment.
¶ Compared with the lignocellulose treatment.
In weaned piglets, Metzler-Zebeli et al. (Reference Metzler-Zebeli, Zijlstra and Mosenthin35) studied the influence of dietary oat β-glucan and CaP level on intestinal microbial composition and metabolic activity. The piglets were fed one of four different diets: maize starch–casein-based diets with a low-CaP or a high-CaP level, or the low- and high-CaP diets supplemented with 8·95 % oat β-glucan concentrate. After slaughter, digesta samples from the ileum, caecum and mid-colon were analysed for bacterial composition, butyrate production pathway genes (quantitative PCR) and fermentation endproducts. The piglets fed the diet with the high CaP level showed a decreased lactate concentration and decreased Streptococcus spp. gene copy numbers in the stomach, as well as a decreased propionate concentration in the large intestine. The caecal gene copy numbers of Clostridium cluster XIVa were increased for piglets fed the high-CaP diet. Similarly, Metzler-Zebeli et al. (Reference Metzler-Zebeli, Vahjen and Baumgärtel124) found elevated gene copy numbers of Clostridium cluster XIVa in the distal ileum of growing pigs that were fed phytase-supplemented maize–soyabean meal-based diets in comparison with a low-P control diet(Reference Metzler-Zebeli, Vahjen and Baumgärtel124). This was associated with the increased availability of phytate-P in the small intestine due to the activity of phytase(Reference Metzler, Mosenthin and Baumgärtel125), and probably with a changed pattern of InsPs in the ileum. Similarly, Blaabjerg et al. (Reference Blaabjerg, Jørgensen and Tauson87) found a decreased InsP6 concentration and a variable InsP pattern in ileal digesta of pigs with increasing dietary levels of plant or microbial phytase. Metzler-Zebeli et al. (Reference Metzler-Zebeli, Zijlstra and Mosenthin35) concluded from the results of their study that the stimulating effect of CaP on the Clostridium cluster XIVa could have been caused by the higher P concentration in the diet, thereby increasing P availability for the microbes. Without oat β-glucan supplementation, high dietary CaP supply decreased gene copies of butyryl-CoA:acetate CoA-transferase in gastric digesta compared with pigs fed the low-P control diet, while this decrease could not be observed for diets supplemented with oat β-glucan. This suggests a positive effect of oat β-glucan on gastric butyrate production, which could be favourable for younger animals such as weaned pigs given the stimulatory effect of butyrate on intestinal development, function and health.
In digesta samples of slaughtered pigs, Varley et al. (Reference Varley, McCarney and Callan119) obtained potential interactions between dietary P content and supplementation of inulin to the diet of pigs with regard to intestinal microbiota composition and activity. Pigs were fed four different wheat–soyabean meal-based diets with either low or high CaP content, each supplemented with inulin (Table 2). No effect of CaP level could be observed in ileal digesta, neither on the specific bacterial groups assessed (i.e. Lactobacillus spp., Bifidobacterium and Enterobacteriaceae) nor on microbial activity as SCFA production was not changed. In contrast, Metzler-Zebeli et al. (Reference Metzler-Zebeli, Mann and Schmitz-Esser36) observed higher numbers of Enterobacteriaceae in the stomach as well as higher numbers of Enterococcus, Bacteroides–Prevotella–Porphyromonas and Campylobacter in the ileum of pigs fed a diet with a high CaP content in comparison with pigs fed a diet with a CaP content according to their actual requirement (Table 2). Ileal Enterobacteriaceae and lactobacilli numbers, however, were not affected. Obviously, effects observed for differences in dietary CaP supply are not only specific for different bacterial species, but are also influenced by sampling site. However, it has to be emphasised that both studies(Reference Metzler-Zebeli, Mann and Schmitz-Esser36, Reference Varley, McCarney and Callan119) differed in the amount and type of substrate available for intestinal fermentation, which could have affected microbial metabolism and composition(Reference Metzler, Mosenthin and Baumgärtel118).
In a study with growing pigs fitted with ileal T-cannulas, Metzler et al. (Reference Metzler, Mosenthin and Baumgärtel125) examined the impact of dietary P and Ca supply, phytase addition and ileal pectin infusion on ileal and faecal P and Ca balance, on chemical composition of faecal MBM, and on the activity of bacterial enzymes. Pigs were either fed a low-P maize–soyabean meal-based control diet and received an ileal infusion of water or pectin once per d, or were fed the control diet supplemented with either monocalcium phosphate (MCP) or supplemented with phytase. MCP supplementation enhanced ileal and faecal P and Ca recovery as well as bacterial incorporation of P and Ca into the MBM, obviously due to an increased intestinal P availability for bacteria(Reference Metzler, Mosenthin and Baumgärtel125). Furthermore, MCP decreased D-+L-lactate in ileal digesta, but did not affect SCFA concentrations in either ileal digesta or faeces. Similarly, Varley et al. (Reference Varley, McCarney and Callan119) observed in growing pigs no effect of variations in P supply on ileal SCFA production as a measure of microbial activity upon feeding diets with varying CaP contents. In the study of Metzler et al. (Reference Metzler, Mosenthin and Baumgärtel125), phytase addition decreased ileal and faecal P recoveries. Reductions in P content in faecal MBM could also be observed, probably due to a lower intestinal P availability for large-intestinal microbes upon phytase supplementation(Reference Metzler, Mosenthin and Baumgärtel125). The decrease in P availability for the intestinal microbes was associated with a tendency towards lowered fermentation activity, similar to observations in studies with rumen microbes(Reference Komisarczuk, Durand and Beaumatin126). In another study of Metzler-Zebeli et al. (Reference Metzler-Zebeli, Vahjen and Baumgärtel124) with growing pigs fitted with simple ileal T-cannulas, the effect of dietary P and Ca supply, phytase addition and ileal pectin infusion on changes in bacterial populations at the ileal and faecal level was assessed. Pigs were either fed a low-P maize–soyabean meal-based control diet and received an ileal infusion of water or pectin once per d, or they were fed a control diet supplemented with either MCP or phytase. Bacterial gene copy numbers determined by quantitative PCR in the ileal digesta of pigs were not different for lactobacilli and Enterobacteriaceae due to MCP supplementation, which is in general agreement with the results of a study by Varley et al. (Reference Varley, McCarney and Callan119) where lactobacilli and Enterobacteriaceae were not influenced upon feeding of a diet with a high CaP content. Metzler-Zebeli et al. (Reference Metzler-Zebeli, Vahjen and Baumgärtel124) also obtained lower ileal gene copy numbers of Enterococcus spp., Enterococcus faecium and the Clostridium leptum cluster due to dietary MCP addition. On the other hand, increasing the P availability in the small intestine through phytase supplementation stimulated the growth of Bacteroides–Prevotella–Porphyromonas, the Clostridium leptum cluster and also the Clostridium coccoides cluster. As phytase addition increased availability of phytate-P but not of Ca, the authors concluded that Ca rather than P might act as a growth-inhibiting factor for specific bacteria, as has been observed for MCP supplementation. The authors suggest that the high level of dietary CaP used in their study may have inhibited the proliferation of specific bacterial groups in the proximal GIT of pigs when compared with pigs fed a control diet without additional CaP supplementation. Increasing the concentration of free Ca2+ ions may reduce the adhesion potential of specific bacterial species, resulting in decreased colonisation of mucosal areas due to competition for the same adhesion sites with other bacterial species(Reference Metzler-Zebeli, Vahjen and Baumgärtel124, Reference Larsen, Nissen and Willats127). Bacterial adhesion to the intestinal mucosa is considered an important factor for bacterial colonisation, as it prevents wash-out of bacteria(Reference Rojas and Conway128, Reference Erickson, Willgohs and McFarland129). However, it needs to be taken into account that Ca plays a significant part in metabolic signalling (such as intracellular signals) in order to affect various cellular processes(Reference Berridge, Bootman and Roderick130) and the composition and activity of the GIT microbiota(Reference Metzler-Zebeli, Mann and Schmitz-Esser36, Reference Mann, Schmitz-Esser and Zebeli131).
Importance of phosphorus for bacterial metabolism with special emphasis on pathogens
The release of P from InsP6 due to supplementation with exogenous phytase plays an important role in improving the P digestibility in plant feed ingredients used in diets for single-stomached animals. However, individual InsPs or phosphate may specifically affect bacterial properties, such as metabolism or virulence. Pathogenic micro-organisms in particular use the host's InsP metabolism to ensure their survival and replication in niches of the GIT(Reference Hilbi25, Reference Weber, Ragaz and Hilbi26). Several enteric pathogens, for example, have developed strategies to metabolically utilise myo-inositol as a carbon and energy source. This feature applies to Gram-positive enteropathogens such as Enterococcus faecalis, Bacillus cereus, Listeria monocytogenes and Clostridium perfringens, but also to Gram-negative pathogens such as Salmonella typhimurium(Reference Staib and Fuchs132) which may under certain circumstances cause infections in pigs. Salmonella typhimurium can utilise myo-inositol as a sole carbon source(Reference Kröger and Fuchs133), and involved genes are suggested to play a role in the Salmonella typhimurium virulence in pigs or chickens(Reference Carnell, Bowen and Morgan134, Reference Chaudhuri, Morgan and Peters135). Other attaching and intracellular pathogenic bacteria interfere with the host's metabolism of InsP to direct their attachment and uptake into phagocytes and non-phagocytic intestinal cells, and to modulate intracellular vesicle trafficking pathways to ensure survival and efficient intracellular replication(Reference McKay and Baird107, Reference Wood and Clark112, Reference Pizarro-Cerdá and Cossart136).
Several intestinal pathogens such as Salmonella spp, enteropathogenic Escherichia coli, Shigella spp. and Yersinia spp. may modulate the host's InsP metabolism in different ways including the production of: (i) InsP-binding proteins to promote efficient host cell entry; (ii) InsP-metabolising enzymes that directly modulate the host cell InsP levels; or (iii) other macromolecules (lipids or proteins) that modulate host cell InsP-metabolising enzymes(Reference McKay and Baird107, Reference Wood and Clark112). For example, Salmonella spp. produces the multifunctional protein SopB (Salmonella outer protein B), a phosphatidylinositol polyphosphatase. SopB triggers the uptake, and maintains high levels of InsP3 in the intracellular Salmonella vacuole, thus enabling intracellular survival and replication(Reference Zhou, Chen and Hernandez137, Reference Marcus, Knodler and Finlay138). In addition, SopB-mediated hydrolysis of InsPs might cause a disruption of epithelial tight junctions, destruction of epithelial integrity and other alterations of membrane integrity leading to fluid secretion, inflammation and diarrhoea(Reference Mason, Mallo and Terebiznik139, Reference Boyle, Brown and Finlay140).
Other mechanisms of pathogens using the metabolism of InsP include InsP6-induced auto-processing of toxins. This mechanism is used by the human pathogens Clostridium difficile and Vibrio cholerae as well as by the porcine pathogen Clostridium perfringens as part of a complex strategy for toxin activation and subsequent delivery of effectors to the target cells. For this purpose, the toxins possess an intrinsic proteolytic activity mediated by an internal cysteine protease domain that may be directly activated by the binding of InsP6(Reference Reineke, Tenzer and Rupnik141–Reference Guttenberg, Hornei and Jank144). Although most of these studies have not been performed with pigs, similar mechanisms may apply for this species. However, despite these fundamental studies on the complex interactions between several pathogens and the host's InsP metabolism, it is still not clear to what extent the dietary modulation of intestinal P and InsP metabolism might influence the metabolism and virulence of intestinal pathogens. Given the high variability of InsP concentration and different forms of InsPs observed in the ileal digesta and faeces of pigs due to different dietary treatments(Reference Blaabjerg, Nørgaard and Poulsen86, Reference Blaabjerg, Jørgensen and Tauson87, Reference Blaabjerg, Hansen-Møller and Poulsen145), further work should focus on the impact of individual InsPs on the properties of intestinal pathogens.
Interactions between dietary phosphorus, intestinal microbiota and host health
Dietary supplementation with P and also Ca has been suggested as a promising strategy to modulate the intestinal eubiosis of pigs(Reference Mann, Schmitz-Esser and Zebeli131). This concept is based on studies with rats, where an improved colonisation resistance against intestinal pathogens and a promotion of lactobacilli in ileal digesta and at the ileal mucosa have been observed for CaP-rich diets(Reference Bovee-Oudenhoven, Wissink and Wouters146). In pig nutrition, dietary CaP contents ranging above pigs’ actual requirements are considered to be disadvantageous. This applies especially for the health of piglets, as excessive CaP supply may compromise gastric barrier function(Reference Lawlor, Lynch and Caffrey147). Evidence that dietary CaP may modulate the porcine intestinal microbiota(Reference Metzler-Zebeli, Zijlstra and Mosenthin35, Reference Metzler-Zebeli, Vahjen and Baumgärtel124) is emerging, although results are not always consistent. For example, the proliferation of several bacterial groups (for example, Enterococcus, Bacteroides–Prevotella–Porphyromonas and Campylobacter) was stimulated in the ileum of pigs upon feeding a high-CaP diet(Reference Metzler-Zebeli, Mann and Schmitz-Esser36), while another study found lower ileal gene copy numbers of Enterococcus spp., Enterococcus faecium and the Clostridium leptum cluster upon dietary MCP addition(Reference Metzler-Zebeli, Vahjen and Baumgärtel124). While the present review focuses on the effects of variations in dietary P supply, it is often difficult to differentiate between effects of P and Ca. Here, specific effects of Ca, such as the potential of free Ca2+ ions to inhibit the proliferation of specific bacterial groups in the proximal part of the GIT, have to be considered(Reference Metzler-Zebeli, Vahjen and Baumgärtel124, Reference Larsen, Nissen and Willats127). This might be of specific importance with regard to competition for adhesion sites and thus for the colonisation of the mucosa and, as a consequence, for the colonisation resistance against potential pathogens. Moreover, several studies(Reference Metzler-Zebeli, Mann and Schmitz-Esser36, Reference Varley, McCarney and Callan119, Reference Metzler-Zebeli, Vahjen and Baumgärtel124) failed to detect any effect of variations in dietary P and Ca content on lactobacilli in ileal and faecal samples. In a recent study by Mann et al. (Reference Mann, Schmitz-Esser and Zebeli131), however, feeding CaP-rich diets to pigs promoted Lactobacillus proliferation, including Lactobacillus mucosae, at the mucosa of the gastric pars non-glandularis. Such a promotion of Lactobacillus mucosae, which is strongly associated with the mucus layer of the intestinal mucosa in pigs(Reference De Lange, Pluske and Gong148), could make a particular contribution to a reduced attachment of opportunistic pathogens by steric hindrance of attachment sites. Generally, Lactobacillus is said to suppress the growth of potential pathogens, such as enterotoxigenic Escherichia coli, through very effective bacteriocin and organic acid production(Reference Chauvière, Coconnier and Kernéis149–Reference Walter, Britton and Roos151). Thus, CaP-related promotion of Lactobacillus might be beneficial in supporting the gastric barrier.
Several studies have shown interactions between dietary CaP supply, fermentation activity and microbial composition of the GIT in pigs(Reference Metzler-Zebeli, Zijlstra and Mosenthin35, Reference Metzler-Zebeli, Mann and Schmitz-Esser36, Reference Metzler, Vahjen and Baumgärtel120). However, studies with pigs focusing not only on potential relationships between dietary P, but also on Ca supply and the animals’ immune system are rare. At least with regard to the expression of pro-inflammatory cytokines, a relationship between dietary P content and the inflammatory response in the small intestine of pigs post-weaning could be established(Reference Metzler-Zebeli, Gänzle and Mosenthin96). Given the close relationship between specific microbial metabolites (for example, butyrate) and expression of cytokines in weaned pigs(Reference Metzler-Zebeli, Gänzle and Mosenthin96), interactions between intestinal microbiota including their metabolic activities, the integrity of the epithelial barrier function, the immune system, and the P content of the diet can be assumed. Furthermore, results from other vertebrate species indicate that dietary deficiency of P may negatively affect lymphocyte function, cytokine secretion and antibody production. Stratification and compartmentalisation, for example, are the main immunological mechanisms preventing the host from colonisation with intestinal pathogens, and both strongly depend on IgA production and cytokine secretion by intestinal immune cells. In an immune-compromised host, however, these mechanisms might be reduced. Reduced lymphocyte function, as a consequence of low P availability, might therefore negatively affect the host's ability to prevent colonisation with intestinal pathogens.
Conclusions
P is an essential nutrient of a porcine diet to maintain health and performance, particularly in the growing pig during the first weeks after weaning. There is rising scientific evidence that P has to be considered as part of an integrated approach to support digestive and immune functions, but can also establish and maintain a stable microbial ecosystem in the GIT with special focus on providing a barrier against potential intestinal pathogens. Although results on the interactions between P and the immune system are inconsistent, several studies have shown a positive impact of dietary P and phytase addition on the adaptive immune response (for example, lymphocyte proliferation, antibody response). The close relationship between the availability of fermentation substrates and available P has to be considered when formulating diets in support of a stable intestinal microbial ecosystem. Differences in P availability and the formation of individual InsPs due to variations in phytate-P content and in the activity of intrinsic phytase (for example, as a consequence of feed processing) of plant feedstuffs also have to be taken into account. Nevertheless, further research into the role of P for the immune system and for the intestinal microbiota is still required, with special focus to be directed to the purpose of individual InsPs for the immune functions of the host, but also for bacterial metabolism. As pathogenic micro-organisms have been shown to use the host's InsP metabolism to ensure their survival and replication in the GIT, future research should also concentrate on the role of dietary P for intestinal pathogens.
Acknowledgements
We thank Sonja Heinritz, Hanna Spindler and Christine Frasch for their assistance in preparing the manuscript.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. C. M. E. H. is supported by the Ministry of Science, Research and Art of the State Baden-Württemberg. The Ministry of Science, Research and Art of the State Baden-Württemberg had no role in the design, analysis or writing of this article.
E. W., S. S., M. R., L. E. H., R. M. and V. S. are responsible for the conceptualisation and implementation of the manuscript. C. M. E. H. wrote the manuscript. All authors reviewed the manuscript and approved submission.
There are no conflicts of interest.