Introduction
Fasting is defined as voluntary abstention from food for varying duration(Reference Trepanowski and Bloomer1), and it has been positively associated with longevity and boosting of human health(Reference Trepanowski, Canale, Marshall, Kabir and Bloomer2). Religious fasting (RF) is a nutritional model that has various levels of food restrictions as a dietary regimen of most popular religions(Reference Lazarou and Matalas3). The possible impact of RF on human health has been the subject of public debate for thousands of years. Over the last two decades, an increasing number of studies have been carried out on RF and its impact on human health. Evidence suggests that RF may have preventive effects on various diseases, such as obesity, cardiovascular diseases (CVD) and type 2 diabetes mellitus (T2DM)(Reference Trepanowski and Bloomer1,Reference Trepanowski, Canale, Marshall, Kabir and Bloomer2,Reference Persynaki, Karras and Pichard4) .
The Christian Orthodox Church (COC) suggests that individuals who follow the long-term structured fasting type of diet abstain from meat, dairy products and eggs for 180–200 d annually, while their diet is characterised by increased consumption of cereals, legumes, fruits, vegetables, fish and seafood. COC fasting includes three main fasting periods: 40 d prior to Christmas (from 15 November to 24 December), 48 d prior to Easter (from Clean Monday to Holy Saturday), 14 d prior to Assumption (from 1 to 14 August), the fasting period prior to the feast of Holy Apostles (lasting 0–30 d depending on Easter feast), and three other daily feasts (5 January, 29 August, 14 September), as well as every Wednesday and Friday(Reference Lazarou and Matalas3,Reference Rodopaios, Manolarakis, Peppas and Kafatos5) . Details about foods to be avoided and the duration of fasting periods can be found in Table 1.
Table 1. Fasting periods according to COC fasting regimes

COC fasting has unique dietary recommendations demonstrating an infrequent interchange from a mixed diet to a vegetarian diet that includes fish and seafood(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6). The periodic fasting followed by Orthodox Christians in Greece during all COC fasting periods resembles the traditional Greek diet, with plenty of legumes, vegetables, fruits, nuts, olives, bread, fish and seafood, such as shrimps, octopus and snails(Reference Lazarou and Matalas3–Reference Rodopaios, Manolarakis, Peppas and Kafatos5,Reference Koufakis, Karras, Antonopoulou, Angeloudi, Zebekakis and Kotsa7,Reference Trichopoulou, Kouris-Blazos and Wahlqvist8) . Since the results of the Seven Countries Study (SCS), the Mediterranean diet and more specifically the diet of Crete have been identified as a healthy diet associated with CVD protection and promoting longevity(Reference Keys, Menotti and Karvonen9). The population of Crete of the SCS had the lowest CHD mortality and the highest life expectancy in comparison with all other fifteen cohorts(Reference Hatzis, Sifaki-Pistolla and Kafatos10). The term Mediterranean diet was generated from the SCS and the diet of Crete in 1960 when 60 % of Greek participants fasted strictly during all fasting periods of the COC(Reference Hatzis, Sifaki-Pistolla and Kafatos10–Reference Sarri and Kafatos12). The longest adult life expectancy and the lowest mortality rates from all chronic diseases seem to be related to the excellent dietary pattern of the population of Crete and the daily intense physical activity(Reference Sarri and Kafatos12,Reference Cannon13) . This lifestyle of the population of Crete in the 1960s resulted in the lowest body weight in comparison with all other fifteen cohorts of the SCS during the 50 years of follow-up(Reference Hatzis, Papandreou and Patelarou14). Also, between 1994 and 1997, the European Prospective Investigation into Cancer (EPIC) study was undertaken in Greece with 28 572 individuals aged 20–86 years participating. After a mean follow-up of 8·5 years, lower overall mortality was found in comparison with that of to the individuals with closer adherence to the traditional Mediterranean diet,(Reference Trichopoulou, Bamia and Trichopoulos15) and as it was observed, COC fasting was followed by 50 % and 65 % of men and women, respectively, who participated in the Greek cohort of EPIC(Reference Lazarou and Matalas3).
Metabolic syndrome (MetS) has become a public health concern and, as defined by the World Health Organization (WHO), is a cluster of disorders that includes abdominal obesity, insulin resistance, dyslipidaemia and hypertension(Reference Saklayen16). Different definitions of MetS from several organisations exist, with the most commonly used for health care plans coming from the WHO(Reference Alberti and Zimmet17), the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III)(18) and the International Diabetes Federation (IDF)(Reference Alberti, Zimmet and Shaw19). Criteria for clinical diagnosis of the MetS are (i) elevated waist circumference with cut-off points according to population- and country-specific definitions, (ii) elevated triglycerides with cut-off point ≥150 mg/dL, (iii) reduced HDL-cholesterol with cut-off points <50 mg/dL in females and <40 mg/dL in males, (iv) elevated blood pressure with cut-off points ≥130 mmHg for systolic and/or ≥85 mmHg for diastolic blood pressure and (v) elevated fasting glucose with cut-off point ≥100 mg/dL(Reference Alberti, Eckel and Grundy20). Prevalence of MetS is increasing rapidly throughout the world, and having it increases the risk of developing CVD twofold and T2DM fivefold(Reference Alberti, Eckel and Grundy20). The prevalence of MetS in Greece according to the METS-GREECE Multicentre Study is 23·6 %(Reference Athyros, Mikhailidis and Papageorgiou21), and according to the ATTICA study is 19·8 %(Reference Panagiotakos, Pitsavos and Chrysohoou22), with both studies using the NCEP ATP III definition for MetS prevalence.
The NCEP ATP III and the American Heart Association have recommended a diet approach to prevent MetS as part of a multifaceted lifestyle approach to reduce CVD risk(23). Part of this approach is being physically active by following the national guidelines for moderate- and/or high-intensity physical activity, as it decreases chronic disease mortality(Reference Stensvold, Viken and Steinshamn24). Among the 1627 participants of the SUN cohort study that were followed up for 6 years, those with the highest adherence to the Mediterranean food pattern had the lowest levels of all risk factors of MetS(Reference Tortosa, Bes-Rastrollo, Sanchez-Villegas, Basterra-Gortari, Nuñez-Cordoba and Martinez-Gonzalez25). Similar results were demonstrated by the PREDIMED study, as it was suggested that adherence to a Mediterranean diet had beneficial effects on the reversion of MetS. Among 5801 individuals, aged 55–80 years, with a 4·8 year follow-up, the ones having the highest adherence to the Mediterranean diet had a 56 % lower likelihood of having MetS(Reference Babio, Bulló and Basora26,Reference Babio, Toledo and Estruch27) . Also, the ATTICA study revealed that, in a population of 2282 individuals over 18 years old who were followed for approximately 8·5 years, those who had higher adherence to the Mediterranean diet reduced their odds of having MetS by 20 %(Reference Panagiotakos, Pitsavos and Chrysohoou22).
Overall, there is growing scientific evidence of how the Mediterranean diet affects MetS risk factors, and limited evidence on the association between COC fasting dietary recommendations and MetS. This scoping review aimed to explore the evidence on the association between the COC long-term structured fasting and various aspects of human health and MetS risk factors. To our knowledge, no study has investigated the effects of the long-term fasting recommendation of the COC diet on MetS. Existing gaps in the available literature and future research are also discussed.
Methods
This scoping review was conducted to find evidence on the association between COC fasting and MetS risk factors. We aimed to respond to this significant research question in a comprehensive manner via the scoping review method. This research method does not include a risk of bias of the evidence, as it reports evidence from existing studies and identifies gaps in the available literature(Reference Munn, Peters, Stern, Tufanaru, McArthur and Aromataris28). The scoping review was made in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines(Reference Moher, Liberati, Tetzlaff and Altman29), and the protocol is presented in detail below. PRISMA 2009 Checklist is included as supplementary material.
Literature search
The authors performed an extensive literature search for published articles from February until March 2020 from the following datasets: PubMed (via MEDLINE), CINAHL, Scopus and Google Scholar. An additional search was performed in June 2020 for new available publications.
To retrieve relevant literature, the search strategy involved the following keywords with any possible combination in the abstracts of the articles: ‘religious fast’ or ‘religious fasting’ or ‘Christian Orthodox fast’ or ‘Christian Orthodox Church fasting’ or ‘Greek Orthodox fast’ or ‘Greek Orthodox fasting’ or ‘Greek Orthodox Church fasting’ or ‘Greek monks’ and ‘health’ or ‘obesity’ or ‘blood lipids’ or ‘cardiovascular diseases’ or ‘metabolic syndrome’.
Inclusion and exclusion criteria
Criteria for the included studies were the following: (i) studies on humans; (ii) studies with anthropometric measurements, such as body weight and waist circumference; (iii) studies with nutrition results including food choices; (iv) studies with biochemical metabolic markers including total cholesterol, HDL-cholesterol, LDL-cholesterol, triacylglycerol, HbA1c and blood pressure levels; (v) studies published in English and Greek language; (vi) studies published from January 1990 to March 2020; and (vii) full-length papers. On the other hand, reviews, editorials, meta-analyses, animal studies, study protocols, abstracts and unpublished results were initially excluded from the review process. Additionally, studies were excluded if focused religion was different than COC and if reported measurements were not health related. No restrictions were made regarding study sample and/or fasting duration.
Data collection and analysis
After duplicate publications removal, abstracts of full publications were assessed for eligibility in this review, in accordance with the inclusion and/or exclusion criteria. Data extracted from each publication are: name of the first author, publication year, aim of the study, study design, country of study, sample size and characteristics of participants, fasting periods, results concerning anthropometric measurements, arterial blood pressure and haematologic data. No meta-analysis was performed due to heterogeneity of studies and reported outcomes.
Results
Eligible articles
The initial search of the databases identified 1169 articles, and 35 more articles were found through manual searching. A total of 286 duplicates were removed, leaving 918 articles to be considered for inclusion after title and abstract screening. Fifty-one full publications were reviewed for eligibility, with twenty publications finally included in our scoping review. The process for identifying and selecting papers for this scoping review is in accordance with PRISMA guidelines and presented in the PRISMA flow diagram in Fig. 1. We also used the quality assessment tool as proposed by Hawker and colleagues(Reference Hawker, Payne, Kerr, Hardey and Powell30) for the included publications, to screen the studies, which can be found in Appendix 1. Results show that most of the publications (18/20) were defined as high quality and only a few (2/20) defined as medium quality.

Fig. 1. Flow chart diagram according to the PRISMA guideline.
In total, twenty publications from eleven unique studies that investigate the relationship between COC fasting and health indices met the eligibility criteria and were finally included in this review. Out of them, two were cross-sectional studies (producing four publications(Reference Rodopaios, Mougios and Konstantinidou31–Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34)), one was a case–control study (producing five publications(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38) ) and eight were prospective studies (producing eleven publications(Reference Bethancourt, Kratz and O’Connor39–Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49)).
Overview of the studies and study sample
Most of the data were collected from Greece, a country where the majority of the people are Christian Orthodox, with study sample coming from different origins of Greece: mainly from Crete and Northern Greece, as two studies were conducted in Crete with seven publications(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Papadaki, Valsta and Lampi42,Reference Papadaki, Vardavas, Hatzis and Kafatos43) ; five different studies in Northern Greece produced eight articles(Reference Rodopaios, Mougios and Konstantinidou31–Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34,Reference Liali, Mpirintzis and Vagdatli44–Reference Karras, Koufakis and Petróczi46,Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49) ; and one study in Peloponnese with one published article(Reference Basilakis, Kiprouli and Mantzouranis41). Also, two unique studies were conducted in Egypt with two publications(Reference Elshorbagy, Jernerén and Basta47,Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48) and one in the USA with two different published articles(Reference Bethancourt, Kratz and O’Connor39,Reference Bethancourt, Kratz and O’Connor40) .
In five publications, fasters were people under religious orders, that is, nuns and monks, mainly living in monasteries(Reference Basilakis, Kiprouli and Mantzouranis41–Reference Karras, Persynaki and Petróczi45); in six, fasters were a mix of nuns, monks, priests and laypeople(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Karras, Koufakis and Petróczi46) ; and in nine, fasters were only laypeople(Reference Rodopaios, Mougios and Konstantinidou31–Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34,Reference Bethancourt, Kratz and O’Connor39,Reference Bethancourt, Kratz and O’Connor40,Reference Elshorbagy, Jernerén and Basta47–Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49) . In nine publications, a control group was included in the analysis, with laypeople being non-fasters(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Rodopaios, Mougios and Konstantinidou31–Reference Rodopaios, Manolarakis and Koulouri33,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48) .
In all studies, participants did not suffer from chronic illnesses and/or received medication and/or supplements. The exact number of recruited participants in all available publications cannot be summed, as there is potential overlap of cases and controls in publications conducted by the same research group(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Rodopaios, Mougios and Konstantinidou31–Reference Rodopaios, Manolarakis and Koulouri33,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Bethancourt, Kratz and O’Connor40,Reference Papadaki, Valsta and Lampi42,Reference Papadaki, Vardavas, Hatzis and Kafatos43,Reference Karras, Persynaki and Petróczi45,Reference Karras, Koufakis and Petróczi46) . Sample size in each publication varies from 1 person(Reference Papadaki, Valsta and Lampi42) to 609 participants(Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34), and target age group varies from 18 to 84 years old in all including articles with the exception of one article focusing on a paediatric population aged 5–15·5 years old(Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34).
Methodology and main outcomes of all COC fasting publications included in this review are displayed in Table 2.
Table 2. Details of COC fasting publications included in the review
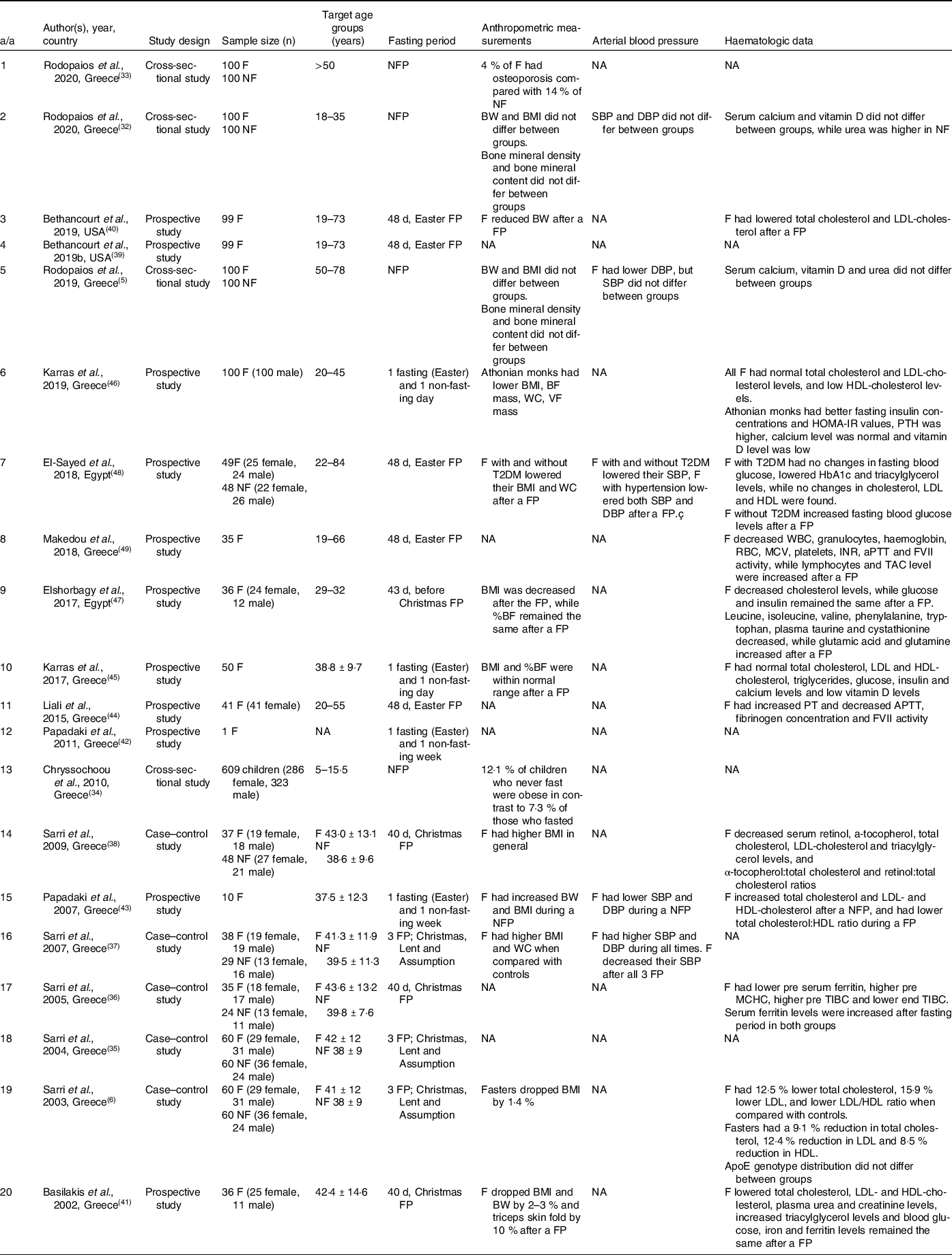
*NA, not applicable; F, faster; NF, non-faster; FP, fasting period; NFP, non-fasting period; BW, body weight; BMI, body mass index; BF, body fat; VF, visceral fat; PT, prothrombin time; APTT, activated partial thromboplastin time; FVII, coagulation factor VII; TAC, total antioxidant capacity.
Frequency of COC fasting practices
Publications included in the analysis vary in duration of examined COC fasting periods between 1 d(Reference Karras, Persynaki and Petróczi45,Reference Karras, Koufakis and Petróczi46) , 7 d(Reference Papadaki, Valsta and Lampi42,Reference Papadaki, Vardavas, Hatzis and Kafatos43) and longer fasting periods(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Liali, Mpirintzis and Vagdatli44,Reference Elshorbagy, Jernerén and Basta47–Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49) . Data on longer COC fasting periods are available from five publications examining the effects of Easter fasting period (48 d)(Reference Bethancourt, Kratz and O’Connor39,Reference Bethancourt, Kratz and O’Connor40,Reference Liali, Mpirintzis and Vagdatli44,Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48,Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49) , four examining the effects of Christmas fasting period (40–43 d depending on the country)(Reference Sarri, Kafatos and Higgins36,Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Basilakis, Kiprouli and Mantzouranis41,Reference Elshorbagy, Jernerén and Basta47) and three focused on three main COC fasting periods, that is, Easter, Christmas and Assumption (totalling 103 d of fasting)(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35,Reference Sarri, Linardakis, Codrington and Kafatos37) . Lastly, five did not focus on specific COC fasting periods, but rather on the effect of adherence to the COC fasting principles(Reference Rodopaios, Mougios and Konstantinidou31–Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34).
Some of them reported the duration of the COC fasting adherence. Fasters in one publication had been observing fast for 14 years(Reference Rodopaios, Mougios and Koulouri32), one reported a mean of 24 ± 10·4 years adherence to the COC fasting rituals(Reference Papadaki, Vardavas, Hatzis and Kafatos43), in others a mean of 20 ± 14 years was mentioned(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38) , in one a mean of 31 years adherence was reported(Reference Rodopaios, Mougios and Konstantinidou31) and in another one a mean of 32 years average fasting time was reported(Reference Rodopaios, Manolarakis and Koulouri33). In the case of participants being under religious orders, one study mentioned 12·5 ± 8·2 years in monastic life(Reference Papadaki, Vardavas, Hatzis and Kafatos43), and in the case of the Athonian monks, 6 months adherence to COC fasting was sufficient to be included in the study(Reference Karras, Persynaki and Petróczi45,Reference Karras, Koufakis and Petróczi46) .
Different dietary assessment methods were used in the publications, and all results are comparable since the methods used are validated. One 24-h dietary recall in combination with a 3-d weighed food record was used in five publications(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38) , three 24-h dietary recalls and a food frequency questionnaire in three publications(Reference Rodopaios, Manolarakis, Peppas and Kafatos5,Reference Rodopaios, Mougios and Koulouri32,Reference Rodopaios, Manolarakis and Koulouri33) , 7-d weighed food records in three publications(Reference Basilakis, Kiprouli and Mantzouranis41–Reference Papadaki, Vardavas, Hatzis and Kafatos43), 7-d weighed food records in combination with a food frequency questionnaire in two publications(Reference Bethancourt, Kratz and O’Connor39,Reference Bethancourt, Kratz and O’Connor40) , 3-d weighed food records in one publication(Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34), 2-d weighed food records in two publications(Reference Karras, Persynaki and Petróczi45,Reference Karras, Koufakis and Petróczi46) and a food frequency questionnaire in one publication(Reference Elshorbagy, Jernerén and Basta47), while three publications did not focus on dietary intake at all(Reference Liali, Mpirintzis and Vagdatli44,Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48,Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49) . Data regarding the dietary assessment method used in each publication and main nutritional outcomes are displayed in Table 3.
Table 3. Results of dietary intake

*NA, not applicable; F, faster; NF, non-faster; FP, fasting period; NFP, non-fasting period; RD, restrictive day; NRD, non-restrictive day.
Blood pressure levels
A few studies have investigated the effect of COC fasting regimes on blood pressure, with mixed results.
According to Sarri and colleagues, after the three main COC fasting periods, mean systolic blood pressure was lowered in thirty-eight fasters when comparing with twenty-nine non-fasters. Further analysis revealed that fasters with high levels of blood pressure, during all fasting periods, were people of older age and with higher BMI, resulting in high levels of blood pressure(Reference Sarri, Linardakis, Codrington and Kafatos37). Also, another study showed that ten members of monasteries in Greece had higher values of systolic blood pressure after an Easter fasting week, probably as a result of lower calcium intake and/or the use of salt during fasting periods to improve the taste of meals(Reference Papadaki, Vardavas, Hatzis and Kafatos43). Elsayed and colleagues reported that, after an Easter fasting period, fasters with and without T2DM decreased systolic blood pressure and increased diastolic blood pressure, and fasters with hypertension lowered both systolic blood pressure and diastolic blood pressure after the fasting period(Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48). A cross-sectional study in Greece demonstrated that both systolic and diastolic blood pressure did not differ between 100 fasters and 100 non-fasters(Reference Rodopaios, Mougios and Koulouri32).
Haematological outcomes
Total cholesterol and LDL-cholesterol decreased by 9·1 % and 12·4 %, respectively, and the LDL:HDL ratio was significantly lower after all three major fasting periods in Greece in sixty fasters who were members of monasteries. This could be a result of the 43·5 % increase in fibre consumption and 17 % reduction in fat intake, as shown from the 24-h recalls and the 3-d weighed food records collected from fasters during all fasting periods. Notably, in the same study, when fasters returned to their usual dietary habits, the levels of total cholesterol and LDL-cholesterol were increased(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6). During an Easter fasting period, in ninety-nine fasters in the United States, it was reported that each serving decrease in meat, dairy product and egg consumption, foods not allowed in this period, was associated with an average decrease of 3·7 % in total cholesterol levels and 3·6 % decrease in LDL-cholesterol(Reference Bethancourt, Kratz and O’Connor40). Also, Papadaki and colleagues showed that, after an Easter fasting week, there were lower levels of total cholesterol and LDL-cholesterol, and the total-cholesterol:HDL-cholesterol ratio was lower, probably as a result of increased fruit, vegetable, legume, fish, shellfish, snail and nut consumption as it was reported in 7-d weighed food records during the fasting period(Reference Papadaki, Vardavas, Hatzis and Kafatos43). Furthermore, total cholesterol(Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Basilakis, Kiprouli and Mantzouranis41,Reference Karras, Koufakis and Petróczi46,Reference Elshorbagy, Jernerén and Basta47) and LDL-cholesterol(Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Basilakis, Kiprouli and Mantzouranis41,Reference Karras, Koufakis and Petróczi46) were both decreased after different COC fasting periods, while HDL was found to be increased during a restrictive period(Reference Karras, Koufakis and Petróczi46) and lower after the Christmas fasting period(Reference Basilakis, Kiprouli and Mantzouranis41).
Triacylglycerol levels were reduced in fasters with T2DM(Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48) and in laypeople following the Christmas fasting period(Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38), while one publication showed that triglycerides were not affected in addition to no changes in glucose metabolism and C-reactive protein levels (CRP)(Reference Bethancourt, Kratz and O’Connor40).
Elsayed and colleagues focused their study on people with and without T2DM. Fasters with T2DM had no changes in fasting blood glucose, while some fasters without T2DM increased their fasting blood glucose levels after the fasting period. Some fasters with T2DM dropped their HbA1c levels, and no changes were found in total cholesterol or LDL- and HDL-cholesterol levels(Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48).
After the Easter fasting period, blood sample analysis showed reduced leucine, isoleucine, valine, phenylalanine, tryptophan, taurine, cystathionine, glutamic acid and glutamine concentrations in fasters(Reference Elshorbagy, Jernerén and Basta47). In addition, plasma retinol and α-tocopherol concentrations, as well as α-tocopherol:total cholesterol and retinol:total cholesterol ratios were all reduced after an Easter fasting period(Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38). According to Basilakis et al., plasma protein, urea and creatinine levels were decreased and blood glucose remained the same, while iron and ferritin levels did not change in fasters after the Christmas fasting period(Reference Basilakis, Kiprouli and Mantzouranis41). Higher serum ferritin levels were also noted after the Christmas fasting period in both fasters and non-fasters(Reference Sarri, Kafatos and Higgins36). In the same study, total iron-binding capacity (TIBC) and mean corpuscular Hb concentration (MCHC) were decreased in fasters(Reference Sarri, Kafatos and Higgins36).
In a study focused on fifty Athonian monks, results showed that they had better fasting insulin concentrations and HOMA-IR values, parathyroid hormone levels were higher, serum calcium was in normal range and all monks were vitamin D deficient. This was probably a result not only of the dietary intake, which was low in vitamin D, but also because of the mandatory clothing of a black cassock they have to wear during outdoor activities(Reference Karras, Persynaki and Petróczi45).
Coagulation factor VII (FVII) activity was found to be lower after a fasting period(Reference Liali, Mpirintzis and Vagdatli44,Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49) . Also, after Easter fasting, prothrombin time (PT) was increased, while activated partial thromboplastin time (APTT) and fibrinogen concentration were lower when comparing with pre-fast values(Reference Liali, Mpirintzis and Vagdatli44). Another publication reported that, after Easter fasting, there was a decrease in leucocytes, granulocytes, haemoglobin, erythrocytes, mean erythrocyte volume (MCV) and platelets, while total antioxidant capacity (TAC) levels were increased(Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49).
Lastly, one study found that ApoE genotype distribution did not differ between fasters and controls after different fasting periods(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6).
Anthropometric parameters
Following the Christmas fasting regimen, fasters dropped their body weight and BMI by 2–3 %, while the triceps skinfold was reduced by 10 %(Reference Basilakis, Kiprouli and Mantzouranis41). Among a Greek population following the three major fasting periods, there was a 1·4 % decline in fasters’ BMI(Reference Sarri, Linardakis, Codrington and Kafatos37). Body weight was found to be lower in fasters when compared with non-fasters after a fasting period(Reference Bethancourt, Kratz and O’Connor40,Reference Papadaki, Vardavas, Hatzis and Kafatos43,Reference Elshorbagy, Jernerén and Basta47) , with same outcomes in BMI(Reference Papadaki, Vardavas, Hatzis and Kafatos43,Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48) , waist circumference(Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48) and lean body mass(Reference Elshorbagy, Jernerén and Basta47). In the only study with children participants, obesity was reported in 12·1 % of those who never fasted in contrast to 7·3 % of children fasters(Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34).
Rodopaios and colleagues reported that overweight and obese participants were over 50 % in both fasters and non-fasters, an outcome that needs further investigation regarding the quantity of foods eaten during fasting periods apart from the quality(Reference Rodopaios, Mougios and Konstantinidou31). Also, in the same study, it was shown that bone mineral density and bone mineral content at the lumbar spine, right and left hip, and right and left femoral neck did not differ between fasters and non-fasters when evaluated with dual-energy X-ray absorptiometry(Reference Rodopaios, Mougios and Konstantinidou31,Reference Rodopaios, Mougios and Koulouri32) , which revealed a 4 % incidence of osteoporosis in fasters compared with 14 % in non-fasters, and bone mineral density was high in overweight and obese men and postmenopausal women over the age of 50 years who followed the COC fasting regimen, without the use of dietary supplements(Reference Rodopaios, Manolarakis and Koulouri33).
Discussion
This scoping review aimed to present available evidence regarding the impact of COC fasting on human health in relation to MetS. As it was shown in some publications, fasting periods were characterised by less daily energy intake when comparing with non-fasting periods(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35,Reference Sarri, Kafatos and Higgins36,Reference Bethancourt, Kratz and O’Connor39,Reference Basilakis, Kiprouli and Mantzouranis41,Reference Papadaki, Valsta and Lampi42,Reference Karras, Koufakis and Petróczi46) , with some reporting a 10 %(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6) or even a 20 %(Reference Basilakis, Kiprouli and Mantzouranis41) reduction in energy intake. Low fat intake(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Rodopaios, Manolarakis and Koulouri33,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35,Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Basilakis, Kiprouli and Mantzouranis41–Reference Papadaki, Vardavas, Hatzis and Kafatos43) , low saturated and trans fatty acid intake(Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35,Reference Papadaki, Valsta and Lampi42,Reference Papadaki, Vardavas, Hatzis and Kafatos43) , and low monounsaturated fatty acid and high ω-3 and ω-6 fatty acids(Reference Papadaki, Valsta and Lampi42) intakes were shown for fasters following COC fasting regimes in different fasting periods. Increased complex carbohydrate and fibre(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Rodopaios, Mougios and Koulouri32,Reference Rodopaios, Manolarakis and Koulouri33,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35,Reference Sarri, Kafatos and Higgins36,Reference Bethancourt, Kratz and O’Connor40) intakes were noted in fasters. In terms of protein consumption, results were mixed; increased protein intake was noted in Athonian monks, probably due to the high intake of shellfish, seafood and snails(Reference Karras, Persynaki and Petróczi45,Reference Karras, Koufakis and Petróczi46) , and in fasters living in the United States, where an increase in plant protein was noted during fasting periods due to high intake of legumes, nuts and seeds(Reference Bethancourt, Kratz and O’Connor39), while lower protein intake was shown in other studies(Reference Rodopaios, Mougios and Konstantinidou31–Reference Rodopaios, Manolarakis and Koulouri33,Reference Sarri, Kafatos and Higgins36,Reference Papadaki, Vardavas, Hatzis and Kafatos43) .
Also, it was shown that people who fasted according to COC recommendations(Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Sarri, Linardakis, Codrington and Kafatos37,Reference Papadaki, Valsta and Lampi42) had increased consumption of fruits, legumes and vegetables that had been associated with a protective effect against CVD, diabetes(Reference Hermsdorff, Zulet, Puchau and Martínez50) and blood pressure(Reference Perez-Vizcaino, Duarte, Jimenez, Santos-Buelga and Osuna51). In a study with 486 females living in Tehran, Esmaillzadeh and colleagues showed that those with the highest intake of fruits and vegetables had a 34 % and 30 % reduced chance of having MetS, respectively, when comparing with those who did not consume the above foods(Reference Esmaillzadeh, Kimiagar, Mehrabi, Azadbakht, Hu and Willett52). Also, as shown in the EPIC-InterAct prospective cohort study, with a representative sample of 16 154 participants and 11 years of follow-up, consumption of a great variety of root and green leafy vegetables was associated with lower risk of diabetes in Europe(Reference Cooper, Forouhi and Ye53). In the PREDIMED study, among 808 participants with high cardiovascular risk, those who had the highest adherence to the Mediterranean diet had 56 % lower odds ratios of having MetS(Reference Babio, Bulló and Basora26). Furthermore, research from the Singapore Chinese Health Study, with 43 176 participants and 11 years of follow-up, indicated that a dietary pattern with high intake of fruits, vegetables and soy products is linked with lower risk of diabetes, while a dietary pattern characterised by higher consumption of meat and processed meat has been linked with higher risk of diabetes in China(Reference Odegaard, Koh and Butler54). As far as the current popular intermittent fasting is concerned, limited evidence exist about its effects in people with diabetes; most of its results are similar to those of caloric restrictions, and it should be followed only with the involvement of a physician, according to a recent review by Horne and colleagues(Reference Horne, Grajower and Anderson55).
High fibre consumption has been observed to be associated with beneficial effects on waist circumference and HDL-cholesterol, both components of MetS(Reference Sahyoun, Jacques, Zhang, Juan and McKeown56). Notably, in the ATTICA study, the Mediterranean diet was associated with 13 % lower likelihood of having the MetS, as shown in 3042 participants in Greece(Reference Panagiotakos, Pitsavos, Skoumas and Stefanadis57). Olive oil, which is allowed during some fasting periods, has been proven to have beneficial effects on lowering blood pressure, reducing plasma glucose and improving the total-cholesterol:HDL-cholesterol ratio, all different variables of MetS(Reference López-Miranda, Pérez-Jiménez and Ros58). Nuts have been shown to reduce plasma cholesterol, triacylglycerol and waist circumference, all being variables of MetS(Reference Alvarez León, Henríquez and Serra-Majem59). Lastly, another study has shown that 84 g of nut consumption per day has beneficial effects on body fat levels and body weight reduction(Reference Wien, Sabaté, Iklé, Cole and Kandeel60).
In addition, findings from the COC fasting studies are similar to Adventist Health Study 2 (AHS-2) results, in which 38 118 strict, lacto-ovo-, pesco- and semi-vegetarians and 33 634 non-vegetarians were included. AHS-2 results revealed that vegetarians had reduced calorie intake, and had higher total fibre and plant protein intake as a consequence of increased fruit, vegetable, legume and nut consumption, and higher intake of ω-3 fatty acids due to consumption of fishes, when compared with non-vegetarians. Results have shown that vegetarians have a significantly lower risk of having MetS when compared with non-vegetarians(Reference Rizzo, Jaceldo-Siegl, Sabate and Fraser61,Reference Rizzo, Sabaté, Jaceldo-Siegl and Fraser62) . Similarly, 9850 adults over 19 years old participated in the Korean National Health and Nutrition Examination Survey, where it was shown that a dietary pattern that included grains, vegetables and fish was associated with a 14 % reduced risk of MetS(Reference Kim and Jo63). One recent review and meta-analysis of different dietary patterns showed that adherence to a ‘healthy’ diet pattern, mainly characterised by high consumption of fruits, vegetables, whole grains, poultry, fish, nuts, legumes and low-fat dairy products, significantly decreased MetS risk by 15 %, when compared with a ‘meat/western’ diet pattern, which increased MetS risk by 19 % and was characterised by high intake of red and processed meat, eggs, refined grains and sweets(Reference Fabiani, Naldini and Chiavarini64).
The effects of COC fasting period on blood pressure results were mixed. Two publications reported no changes in both systolic blood pressure and diastolic blood pressure(Reference Rodopaios, Mougios and Konstantinidou31,Reference Rodopaios, Mougios and Koulouri32) , and one showed low levels of systolic blood pressure under all fasting periods(Reference Sarri, Linardakis, Codrington and Kafatos37). Also, Papadaki et al. showed that both systolic blood pressure and diastolic blood pressure were lower only in non-fasting periods(Reference Papadaki, Vardavas, Hatzis and Kafatos43). Taking into consideration that salt intake was never investigated in any study, high blood pressure could be possibly be due to the addition of salt during cooking in favour of taste(Reference Papadaki, Vardavas, Hatzis and Kafatos43). Also, it could be a result of low calcium intake during COC fasting periods, given that calcium has been associated with blood pressure control(Reference Miller, Jarvis and McBean65). In some cases, this could be due to the undiagnosed prevalence of arterial hypertension as people under religious orders living in monasteries might have limited access to health care services(Reference Sarri, Linardakis, Codrington and Kafatos37,Reference Tedesco, Di Salvo and Caputo66) . Results from the SUN cohort study, in 9408 participants with a follow-up of 6 years, showed that people with higher adherence to the Mediterranean diet had lower mean levels of systolic and diastolic blood pressure when compared with those with the lowest adherence in Spain(Reference Núñez-Córdoba, Valencia-Serrano, Toledo, Alonso and Martínez-González67). Similarly, in Greece, the results of the EPIC study indicated that adherence to the Mediterranean diet is inversely associated with blood pressure levels(Reference Psaltopoulou, Naska, Orfanos, Trichopoulos, Mountokalakis and Trichopoulou68), and the ATTICA study showed that a food pattern characterised by consumption of fish, vegetables, fruits, legumes and cereals was inversely associated with blood pressure(Reference Panagiotakos, Pitsavos, Skoumas and Stefanadis57). What is more, among 6627 vegans, vegetarians and fish eaters and 4737 meat eaters participating in the EPIC-Oxford study in the UK, vegans had the lowest prevalence of hypertension and the lowest mean systolic and diastolic blood pressure, while meat eaters had the highest values(Reference Appleby, Davey and Key69). Same findings were shown in 500 non-Black individuals in AHS-2(Reference Pettersen, Anousheh, Fan, Jaceldo-Siegl and Fraser70), as well as in 592 Black individuals in AHS-2 across the United States and Canada(Reference Fraser, Katuli, Anousheh, Knutsen, Herring and Fan71), where vegans had significantly lower values in systolic and diastolic blood pressure when compared with non-vegetarians. In contrast, blood pressure was not different for people following alternate-day fasting, which accounts for one fast day and one usual intake, for 6 and 12 months when comparing with a control group(Reference Trepanowski, Kroeger and Barnosky72). Also, a similar reduction in both systolic and diastolic blood pressure was observed in premenopausal women following intermittent fasting and in those who followed a low-calorie diet(Reference Harvie, Pegington and Mattson73).
A potential benefit for lipid profile is indicated, as total cholesterol and LDL-cholesterol concentrations were reduced at the end of COC fasting periods(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Sarri, Bertsias, Linardakis, Tsibinos, Tzanakis and Kafatos38,Reference Bethancourt, Kratz and O’Connor40,Reference Basilakis, Kiprouli and Mantzouranis41,Reference Papadaki, Vardavas, Hatzis and Kafatos43,Reference Karras, Persynaki and Petróczi45,Reference Elshorbagy, Jernerén and Basta47) . Some studies showed a reduction in HDL-cholesterol concentrations(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Basilakis, Kiprouli and Mantzouranis41,Reference Karras, Koufakis and Petróczi46) , a finding that is common in low-fat and vegetarian diets(Reference Asztalos, Lefevre and Wong74,Reference Wang, Zheng, Yang, Jiang, Fu and Li75) . Similar results were shown in the PREDIMED study, where participants with higher adherence to the Mediterranean diet had 54 % lower odds of having high triacylglycerol levels(Reference Babio, Bulló and Basora26) and 47 % lower odds of having low HDL-cholesterol levels(Reference Babio, Toledo and Estruch27), both MetS criteria. The EPIC-Oxford study, with 11 004 participants in the UK, showed that mean LDL-cholesterol was 12 % lower in vegetarians than in omnivores(Reference Appleby, Davey and Key69). Also, the ATTICA study in Greece, with 3042 participants, showed that the Mediterranean diet was inversely associated with triacylglycerol levels and positively associated with HDL-cholesterol levels(Reference Panagiotakos, Pitsavos, Skoumas and Stefanadis57).
Fasting blood glucose has not been investigated thoroughly, apart from one study with participants having both lower and higher fasting blood glucose levels after a fasting period(Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48). The Tzu Chi Health Study, with 4384 Taiwanese participants, found that vegetarian diet characterised by increased intake of soy products, vegetables, nuts and whole-grain products was negatively associated with impaired fasting blood glucose. The prevalence of diabetes was 0·6 % in vegetarian pre-menopausal women versus 2·3 % in meat eater ones, 2·8 % versus 10 % in vegetarian menopausal women versus meat eaters, and 4·3 % and 8·1 % in vegetarian men and omnivores, respectively(Reference Chiu, Huang and Chiu76). Lower prevalence and incidence of diabetes were also found in 41 387 vegetarians from the United States and Canada who participated in the AHS-2 study(Reference Tonstad, Stewart, Oda, Batech, Herring and Fraser77).
Results concerning body weight and BMI are limited. Studies showed a reduction in body weight(Reference Bethancourt, Kratz and O’Connor40,Reference Basilakis, Kiprouli and Mantzouranis41,Reference Papadaki, Vardavas, Hatzis and Kafatos43) and BMI(Reference Papadaki, Vardavas, Hatzis and Kafatos43,Reference Elshorbagy, Jernerén and Basta47,Reference Elsayed, Noreldin, Elsamman, Zaky and Kaldas48) , while one showed optimal body weight in fasters before and after all fasting periods(Reference Karras, Persynaki and Petróczi45), but others do not report any data, meaning that conclusions cannot be drawn. Similarly, due to the effect of low energy intake in vegetarian diets, people who follow these diets have lower BMI when compared with meat eaters(Reference Key, Appleby and Rosell78). Furthermore, Mediterranean diet seems to contribute to weight loss and maintaining a healthy body weight(Reference Hassapidou, Tziomalos and Lazaridou79,Reference Poulimeneas, Anastasiou, Santos, Hill, Panagiotakos and Yannakoulia80) . Also, results from the EPIC-Oxford(Reference Davey, Spencer, Appleby, Allen, Knox and Key81), AHS-2 study(Reference Rizzo, Jaceldo-Siegl, Sabate and Fraser61) and Tzu Chi Health Study(Reference Chiu, Huang and Chiu76) showed that vegetarians had lower BMI when compared with non-vegetarian people, and these differences were reflected in a lower prevalence of obesity in vegetarians. In general, it has been observed that vegetarians are leaner than omnivores(Reference Spencer, Appleby, Davey and Key82,Reference Newby, Tucker and Wolk83) , probably due to high intake of fibre in these diets(Reference Davey, Spencer, Appleby, Allen, Knox and Key81,Reference Cade, Burley and Greenwood84) . Trepanowski and colleagues showed in a recent randomised clinical trial that participants who followed the strategy of alternate-day fasting did not have a significantly different weight loss after 6 and 12 months when compared with people who did not fast(Reference Trepanowski, Kroeger and Barnosky72).
Although studies regarding COC fasting regimen were carried out in three different countries, that is, Greece, United States and Egypt, the main results were similar and demonstrated low energy intake, low fat intake, low animal protein and high vegetable protein intake, high complex carbohydrate and fibre intake, and low calcium and vitamin D intake in laypeople following the COC fasting guidelines during different fasting periods. It is of great importance to mention that the fifth-largest Orthodox population in the world is found in Greece, followed by Egypt in tenth place and the United States in sixteenth(85). It should also be mentioned that different variants of the COC fasting diet are included in the review, from Cretan and Mediterranean diet where meat consumption is allowed in non-fasting periods, to Athonian diet where meat is not allowed at all, but fish, seafood and snails are allowed. This contributes to the increased protein intake in Athonian monks seen throughout the year(Reference Karras, Koufakis and Petróczi46).
Results from available studies with participants following the COC fasting recommendations revealed some positive outcomes on different variables of the MetS, although conclusions cannot be drawn on individual studies regarding any aspect of health.
Limitations
Our scoping review has several limitations which have to be taken into consideration when results are being evaluated, with some already being mentioned. First, the number of available and included articles is low, with small number of subjects involved from the same research group(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Rodopaios, Mougios and Konstantinidou31,Reference Rodopaios, Mougios and Koulouri32,Reference Sarri, Linardakis, Bervanaki, Tzanakis and Kafatos35–Reference Bethancourt, Kratz and O’Connor40,Reference Papadaki, Valsta and Lampi42,Reference Papadaki, Vardavas, Hatzis and Kafatos43,Reference Karras, Persynaki and Petróczi45,Reference Karras, Koufakis and Petróczi46) . There is lack of control group in eleven out of twenty publications investigating the health effects of COC fasting regimen(Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34,Reference Bethancourt, Kratz and O’Connor39–Reference Elshorbagy, Jernerén and Basta47,Reference Makedou, Vagdatli, Patziarela, Konstantinidou, Poimenidou and Lymperaki49) and the possibility of under- or over-estimating dietary intake either when subjects report it on questionnaires or when investigators analyse the information on available nutrition software in sixteen studies(Reference Sarri, Tzanakis, Linardakis, Mamalakis and Kafatos6,Reference Rodopaios, Mougios and Konstantinidou31,Reference Rodopaios, Mougios and Koulouri32,Reference Chryssochoou, Linardakis, Chatziagorou, Tsanakas and Kafatos34–Reference Papadaki, Vardavas, Hatzis and Kafatos43,Reference Karras, Persynaki and Petróczi45–Reference Elshorbagy, Jernerén and Basta47) , which are among the confounding variables that influence the cause-and-effect relationship we are noticing. Moreover, the time intervals during which fasters were followed were short with no long-term follow-up period in all included articles. Also, the level of adherence and the years of compliance with the COC fasting guidelines were different in most of the included articles. No study was focused on foods eaten during non-fasting periods that might affect the results of studies investigating the long-term results of COC fasting regimen, and heterogeneity was high regarding design method and study sample. Finally, the included studies focus on different risk factors of MetS, meaning that results are interpreted individually and we cannot draw a clear conclusion.
Conclusion
The COC fasting diet pattern could be advised for the prevention of chronic diseases, as well as for people who want to follow a plant-based diet in terms of a healthier and/or more sustainable way of living. As already mentioned, the COC fasting dietary pattern has been the origin and the main characteristic of the diet of Crete since the 1960s, and was later called the Mediterranean diet. Adherence to the traditional diet of Crete is the main way of decreasing the MetS score. Thus, the COC fasting dietary pattern or its most beneficial components together with physical activity may be effective advice to decrease the MetS score.
Although evidence in favour of health benefits is available, the COC fasting dietary recommendations should always be followed under personalised guidance on proper meal planning, like any other dietary pattern. Adding to this, the quality and the quantity of foods consumed during fasting and non-fasting periods have to be examined thoroughly. What is more, long-term follow-up studies should be carried out to investigate if the positive effects on health parameters are sustainable over time.
Further investigation is needed in relation to several health parameters, disease indices that are influenced by diet and in diverse ethnic populations, in order to assess the impact of this type of periodic vegetarianism that includes fish and seafood on nutritional status and chronic diseases. Taking into consideration the significant impact it has on public health, future studies should be focused on the interaction between COC fasting regimen and all risk factors of MetS.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Author contributions
Anna Kokkinopoulou: Conceptualisation, Methodology, Data collection, Data analysis, Data interpretation, Writing original draft, Writing - review & editing, Visualisation, Final approval of the submitted version
Anthony Kafatos: Conceptualisation, Methodology, Data collection, Data analysis, Data interpretation, Writing original draft, Writing - review & editing, Visualisation, Final approval of the submitted version, Supervision
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0954422421000184
Appendix 1 Results of the quality assessment tool for the qualitative studies (n = 20)








