Introduction
During the last few years, a growing number of studies focusing on the developmental origin of health and disease have identified links among early nutrition, epigenetic processes and diseases also in later life. There is convincing experimental evidence to suggest that epigenetic marks serve as a memory of exposure in early life to inadequate or inappropriate nutritional factors. These marks induce long-term changes in gene expression, potentially leading to diseases in adulthood, according to the ‘developmental origin of health and disease’ (known as DOHaD) hypothesis(Reference Burdge and Lillycrop1–Reference Niculescu and Lupu3). Epigenetic modifications may be one mechanism by which exposure to an altered intra-uterine milieu or metabolic perturbation may influence gene expression and modulate the phenotype of the organism much later in life(Reference Simmons4). Exposures during early life may be critical, as the plasticity of growing and developing tissues means that they shape the way in which the body responds to later challenges.
The purpose of this review is to present the latest scientific data suggesting that nutrition in early life could be considered as an important risk factor for non-communicable diseases of adulthood. In this regard, the role of intestinal microflora and its metabolites, such as SCFA and especially butyrate, will be addressed. Probiotics are largely used in human nutrition, especially in the paediatric age. Many infant formulas, and other baby food products contain probiotics. The possible presence of probiotic strains even in human milk is debated too. It is hypothesised that the biological function of probiotics may be the result of epigenetic modifications that may explain the wide range of the observed effects.
Epigenetic mechanisms elicited by nutritional factors
Epigenetics encompasses changes to marks on the genome that are copied from one cell generation to the next, which may alter gene expression, but which do not involve changes in the primary DNA sequence. There are three distinct, but closely inter-acting, epigenetic mechanisms possibly elicited by nutritional factors (Table 1). They include DNA methylation, histone modifications and non-coding microRNAs (miRNA; short RNA molecules) which, together, are responsible for regulating the intensity and the timing of expression of specific genes, not only during cellular differentiation in embryonic and fetal development but also throughout the life-course(Reference McKay and Mathers5, Reference Zeisel6).
Table 1 Epigenetic roles of main nutritional factors and type of diets

Effects of nutritional factors on DNA methylation
Changes in DNA methylation are an essential part of normal development. Nutritional factors can change DNA methylation of gene specific promoters, which are closely associated with gene expression. A proportion of the cytosine residues is modified after translation by attachment of a methyl group to position 5 on the cytosine ring. Such methylated cytosines are usually found where the cytosine is next to a guanine residue, i.e. in a cytosine–phosphate–guanine (CpG) dinucleotide. In about half the genes of human genome, unmethylated CpG are found clustered at the 5′ ends of genes in domains known as CpG islands. When the CpG in such islands are unmethylated, gene transcription proceeds normally but when some or all of the CpG become methylated, the genes are switched off(Reference Mathers7).
Folate has been extensively studied for its effect on DNA methylation, because folate carries a methyl group and ultimately delivers this methyl group for the synthesis of adenosyl methionine (AdoMet), the unique methyl donor for DNA methylation reactions. However, folate is not the unique determinant of DNA methylation, because other methyl donor nutrients such as methionine, choline, betaine, and vitamins B2, B6 and B12 can also change DNA methylation status(Reference Choi and Friso8). Because folate deficiency during pregnancy is associated with an increased risk of neural tube defects, aberrant reprogramming of DNA methylation by low dietary folate has been suggested as a candidate mechanism. In an animal study, restriction of folate, vitamin B12 and methionine from the periconceptional diet induced obesity in adult offspring and an altered immune responses to an antigenic challenge(Reference Steegers-Theunissen, Obermann-Borst and Kremer9). However, periconceptional supplementation of folic acid is associated with imprinting status of insulin-like growth factor 2 in the child, which may affect intra-uterine growth with potential consequences in adulthood as illustrated by the association between higher insulin-like growth factor 2 methylation and decreased birth weight(Reference Sinclair, Allegrucci and Singh10). Choline and betaine, two donors of methyl groups, have been studied for their role upon brain development and function. Zeisel(Reference Zeisel11) demonstrated that choline availability during fetal brain development induces epigenetic changes that can finely modulate the expression of genes with specific roles in neuronal differentiation and angiogenesis in the fetal hippocampus(Reference Zeisel11, Reference Mehedint, Craciunescu and Zeisel12). Dietary restriction in methyl donors as well as genetic polymorphisms in folate metabolism have been associated with abnormal DNA methyltransferase expression, global DNA hypomethylation and increased cancer risk(Reference Kanai and Hirohashi13). Using the agouti viable yellow (Avy) mouse model, Waterland(Reference Waterland14) have also shown that methyl donor supplementation prevents transgenerational amplification of obesity through three generations, suggesting a role for DNA methylation in the developmental establishment of body-weight regulation. All these studies suggest that DNA methylation induced by nutritional factors in early life could play a critical role in development regulation not only during the fetal period but also throughout the life-course. The new research reveals that a new type of epigenetic modification, 5-hydroxymethylcytosine, plays a critical role mediating the external signals that instruct a cell how to develop. Hydroxymethylation appears to be linked to a higher degree of pluripotency, and the balance between hydroxymethylation and methylation in the genome is inextricably linked with the balance between pluripotency and lineage commitment. This new epigenetic mark, hydroxymethylation, might help with developing improved strategies for regenerative medicine(Reference Ficz, Branco and Seisenberger15).
Effects of nutritional factors on histone modifications
In contrast to DNA that is modified only by methylation, histones can be modified by methylation, acetylation, phosphorylation, biotinylation, ubiquitination and ADP-ribosylation, which are different types of chemical modifications mainly located within the N-terminal tails of core histones. Histone acetylation is one of the most extensively studied epigenetic mechanisms. Histone tail acetylation is believed to enhance the accessibility of a gene to the transcription machinery, whereas deacetylated tails are highly charged and believed to be tightly associated with the DNA backbone, thus limiting accessibility of genes to transcription factors(Reference Delage and Dashwood16). Interestingly, histone deacetylase (HDAC) inhibitors have been recognised as new potential therapeutic drugs against cancer, because they induce cell cycle arrest and apoptosis by enhancing the expression of certain pro-apoptotic or cell cycle-mediating genes. Recent interest in HDAC inhibitors has expanded into the realm of cancer chemoprevention, as distinct from cancer therapy, with evidence that dietary compounds such as butyrate, diallyl disulfide and sulforaphane act as weak ligands for HDAC and exhibit HDAC inhibitory activity. The working hypothesis for dietary agents is that DNA/chromatin interactions are kept in a constrained state in the presence of HDAC/co-repressor complexes, but HDAC inhibitors enable histone acetyltransferase/co-activator (HAT/CoA) complexes to transfer acetyl groups to lysine ‘tails’ in histones, thereby loosening the interactions with DNA and facilitating transcription factor access and gene activation. Among the epigenetically silenced genes that have received particular interest are p21 and bax due to their implications for cell cycle arrest and apoptosis, and because they are among a select cadre of genes frequently repressed in cancer cells and de-repressed following treatment with HDAC inhibitors.
Different dietary agents such as butyrate, biotin, lipoic acid, garlic organosulfur compounds, and metabolites of vitamin E have structural features compatible with HDAC inhibition. The ability of dietary compounds to de-repress epigenetically silenced genes in cancer cells, and to activate these genes in normal cells, has important implications for cancer prevention and therapy(Reference Dashwood and Ho17). In a broader context, there is growing interest in dietary HDAC inhibitors and their impact on epigenetic mechanisms affecting other chronic conditions, such as CVD, neurodegeneration and ageing.
Effects of nutritional factors on microRNAs
RNA is not only a messenger operating between DNA and protein. Transcription of the entire eukaryotic genome generates a myriad of non-protein-coding RNA species that show complex overlapping patterns of expression and regulation. miRNA are small RNA molecules encoded in the genome that can have a profound effect in controlling gene expression. miRNA bind to their target mRNA and down-regulate their stabilities and/or translation. Each miRNA is predicted to have many targets, and each mRNA may be regulated by more than one miRNA(Reference Lewis, Shih and Jones-Rhoades18, Reference Lim, Lau and Garrett-Engele19). miRNA can play important roles in controlling DNA methylation and histone modifications, creating a highly controlled feedback mechanism(Reference Chuang and Jones20). Interestingly, epigenetic mechanisms such as promoter methylation or histone acetylation can also modulate miRNA expression. A relationship between epigenetics and miRNA has been found to play important roles in carcinogenesis by altering cell proliferation and apoptosis(Reference Iorio, Piovan and Croce21). Curcumin, genistein and retinoic acid are bioactive food components which are able to reduce carcinogenesis through miRNA(Reference Salerno, Scaglione and Coffman22–Reference Weiss, Marques and Woltering25). More recently, it has been suggested that miRNA are involved not only in carcinogenesis, but also in the genesis of insulin resistance and other related disorders. Animal models of methyl-deficient diet suggest that alterations in the expression of miRNA are a fundamental event during the development of liver cancer and non-alcoholic steatohepatitis induced by dietary methyl deficiency(Reference Pogribny, Starlard-Davenport and Tryndyak26). Alterations of miRNA are also observed in pigs fed a high-cholesterol diet compared with those fed a standard diet, indicating the potential implications of miRNA in obesity(Reference Cirera, Birck and Busk27). Although long non-coding RNA are among the least well understood of non-protein-coding RNA species, they cannot all be dismissed as merely transcriptional ‘noise’. Recent evidence suggests their roles in transcriptional regulation, epigenetic gene regulation and diseases(Reference Ponting, Oliver and Reik28). In this area, more studies are needed to evaluate the therapeutic potential of epigenetic modifiers and non-protein-coding RNA species.
Epigenetic mechanisms elicited by maternal diet during pregnancy
Epidemiological and experimental data obtained in animal models(Reference Godfrey and Barker29–Reference Armitage, Taylor and Poston32) show that both under- and over-nutrition during pregnancy and/or lactation induce stable alterations to the physiological and structural phenotype of the offspring. Studies in animal models have used a candidate gene approach to identify the molecular basis for changes in activities of metabolic and endocrine pathways, with a specific focus on corticosteroid activity, and carbohydrate and lipid metabolism.
In rats moderate maternal dietary protein restriction is known to alter phenotypes in the offspring, which manifests as hypertension, dyslipidaemia and impaired glucose metabolism. However, these abnormalities can be reversed by folate supplementation. It has been shown that the induction of an altered phenotype by a maternal protein-restricted diet during pregnancy involves changes in DNA methylation and histone modifications in specific genes, including the glucocorticoid receptor (33 % lower; P < 0·001) and PPARα (26 % lower; P < 0·05) in the liver of juvenile and adult offspring(Reference Lillycrop, Phillips and Torrens33, Reference Lillycrop, Slater-Jefferies and Hanson34), as well as hepatocyte nuclear factor 4a (Hnf4a) in pancreatic islets(Reference Sandovici, Smith and Nitert35). However, a high protein intake in rats during pregnancy and lactation also results in male offspring with higher blood pressure and female offspring with higher body mass and increased fat pad mass; it is possible to speculate that these effects are also mediated by epigenetic mechanisms(Reference Thone-Reineke, Kalk and Dorn36).
Maurer & Reimer(Reference Maurer and Reimer37) showed that a maternal high-protein diet, but not high-prebiotic fibre diet, during pregnancy and lactation could negatively influence the expression of genes involved in glucose and lipid metabolism in the offspring rats. These early changes, perhaps based on epigenetic mechanisms, could have long-term consequences for the development of obesity and the metabolic syndrome(Reference Maurer and Reimer37).
Interestingly, a number of clinical studies have shown that the highest risk for development of the metabolic syndrome and diabetes occurs in adults who are born small for gestational age and become overweight in early childhood. The association between low birth weight and early postnatal catch up growth with late onset of disease is due to very early development of insulin resistance(Reference Ong38–Reference Cianfarani, Germani and Branca40). In experimental animal studies there is evidence that prenatal under-nutrition causes alteration in pancreatic islet neogenesis, impairing the capacity of β-cell regeneration(Reference Garofano, Czernichow and Bréant41). This may explain the inability of β-cell mass to adapt during ageing, which aggravates glucose tolerance. The rat model of protein restriction showed that maternal low-protein diet results in increased susceptibility to insulin resistance in the offspring and that this effect was attributed to reduced β-cell mass due to lower cell proliferation(Reference Petrik, Reusens and Arany42). Pinney & Simmons(Reference Pinney and Simmons43) studied epigenetic events at the promoter of the gene encoding pancreatic and duodenal homeobox 1 (Pdx-1), a critical transcriptional factor for β-cell function and development, the expression of which is reduced in intra-uterine growth retardation (IUGR), promoting the development of diabetes in adulthood. IUGR resulted in transcriptional repression of Pdx-1 due to histone deacetylation and a consequent loss of binding of major transcription factors to the Pdx-1 promoter. At the neonatal stage, this epigenetic process is reversible and may define an important developmental window for therapeutic approaches. After birth, histone deacetylation progresses and is followed by a marked decrease in histone H3 lysine 4 (H3K4) trimethylation and a significant increase in dimethylation of histone H3 lysine 9 (H3K9) in IUGR islets. H3K4 trimethylation is usually associated with active gene transcription, while H3K9 dimethylation is usually a repressive chromatin mark. Progression of these histone modifications parallels the progressive decrease in Pdx-1 expression which locks in the silenced state in the IUGR adult pancreas resulting in diabetes(Reference Pinney and Simmons43). Similarly, Raychaudhuri et al. (Reference Raychaudhuri, Raychaudhuri and Thamotharan44) demonstrated that perinatal nutrient restriction resulting in IUGR leads to histone modifications in skeletal muscle that directly decrease GLUT type 4 (Glut4) gene expression. This effectively creates a metabolic knockdown of this important regulator of peripheral glucose transport and insulin resistance, thereby contributing to adult type 2 diabetes(Reference Raychaudhuri, Raychaudhuri and Thamotharan44).
Besides prenatal under-nutrition models, the metabolic intra-uterine environment may also be modified in the case of prenatal over-nutrition. There is evidence that increased dietary fat intake during pregnancy and lactation predisposes the offspring to develop a metabolic syndrome-like phenotype in adult life. It has been found that maternal high fat feeding results in the offspring having exacerbated adiposity and modified expression of key proteins involved in hepatic insulin sensitivity(Reference Buckley, Keserü and Briody45). These offspring also develop endothelial, cardiovascular dysfunction and sex-specific hypertension(Reference Khan, Taylor and Dekou46, Reference Khan, Dekou and Douglas47).
Maternal fat intake contributes toward non-alcoholic fatty liver disease progression in adult offspring, which is mediated through impaired hepatic mitochondrial metabolism and up-regulated hepatic lipogenesis. It is plausible to speculate that suboptimal nutrition during the developmental period may alter the epigenetic profile of key genes, subsequently leading to persistent modulation in gene transcription and increasing the risk of developing non-alcoholic steatohepatitis in adulthood(Reference Bruce, Cagampang and Argenton48). Perinatal exposure to high-fat, high-sugar diets also results in altered development of the central mesolimbic reward system. These offspring exhibit increased preference for fat, leading to suggestions that perinatal exposure to high-fat, high-sugar foods results in permanent changes within the central reward system that increase the subsequent drive to overconsume palatable foods in postnatal life(Reference Ong and Muhlhausler49).
Recent evidence indicates that JmjC-domain-containing histone demethylase 2A (JHDM2a), which catalyses removal of H3K9 mono- and dimethylation through Fe- and α-ketoglutarate-dependent oxidative reactions, regulates metabolic genes related to energy homeostasis including anti-adipogenesis, regulation of fat storage, glucose transport and type 2 diabetes. Mice deficient in JHDM2a develop adult-onset obesity, hypertriacylglycerolaemia, hypercholesterolaemia, hyperinsulinaemia and hyperleptinaemia, which are hallmarks of the metabolic syndrome. JHDM2a− / − mice furthermore exhibit fasted-induced hypothermia indicating reduced energy expenditure and also have a higher RQ indicating less fat utilisation for energy production. These observations may explain the obesity phenotype in these mice. Thus, H3K9 demethylase JHDM2a is a crucial regulator of genes involved in energy expenditure and fat storage, which suggests that it represents a previously unrecognised key regulator of obesity and the metabolic syndrome(Reference Inagaki, Tachibana and Magoori50).
Diabetic patients continue to develop inflammation and vascular complications, even when glycaemia is controlled. This process is attributed to a ‘hyperglycaemic metabolic memory’ based on epigenetic mechanisms. Brasacchio et al. (Reference Brasacchio, Okabe and Tikellis51) shows that periods of transient or prior hyperglycaemia lead to various methylation and demethylation events that, when integrated, have an impact on gene activity. These events lead to sustained activation of pro-inflammatory pathways, which are likely to participate in the progression of diabetic complications. Further understanding of the chromatin remodelling events and how they are linked to ongoing vascular changes in diabetes should lead to better strategies to reduce the burden of diabetes complications(Reference Brasacchio, Okabe and Tikellis51).
Microbiota, epigenetics and early postnatal nutrition
It is now becoming clear that the early postnatal environment, including nutrition, is also a vital determinant of adult health (Fig. 1). Environmental exposures such as early infant diet are believed to make an impact on the development and function of gut microbiota(Reference BerniCanani, Passariello and Buccigrossi52). The intestinal microbiota plays a critical role in the establishment and maintenance of healthy immune responses. Delayed colonisation of the infant gut with commensal bacteria or alterations in the microbiota profile are suggested to be strong risk factors for the development of immune-mediated chronic disorders such as allergic and autoimmune diseases(Reference Licciardi, Wong and Tang53). Solid scientific arguments suggest that immune deviances later in life could be the consequence of an inadequate bacterial pressure on the intestinal mucosa at the early stage. A variety of epigenetic modifications taking place in this early stage could account for the deviant programming of later immunity and overall health status (Fig. 2) (Reference Langhendries, Maton and François54). While the role of epigenetics in postnatal programming of the neonate remains to be demonstrated, there appears to be a window in which infants are vulnerable(Reference Wiedmeier, Joss-Moore and Lane55). Restoring the microbiota profile with a single bacteria species may be effective in the prevention or treatment of allergic and inflammatory diseases, but only if this occurs during the neonatal period. These observations have led to the idea that probiotics, which have the potential to restore the intestinal microbiota balance, may be effective in preventing the development of chronic immune-mediated diseases(Reference Licciardi, Wong and Tang53). A probiotic is defined as a living micro-organism which when administered in adequate amounts confers a health benefit on the host(Reference Brown and Valiere56). The exact mechanisms of action for probiotic bacteria have yet to be fully understood, but it is hypothesised that the biological function of probiotics may be a result of epigenetic modifications that may explain the wide range of the observed effects. An important role for probiotic bacteria is the fermentation of dietary compounds leading to production of SCFA. Studies delineating the effects of probiotics on SCFA production and the epigenetic actions of SCFA will assist in understanding the association between microbiota and allergic or autoimmune disorders. The SCFA butyrate, a main endproduct of microbial fermentation of dietary fibres in the human intestine, plays an important role in the maintenance of intestinal homeostasis and overall health status. The effects exerted by butyrate are multiple from the gut to the peripheral issues with a high potential for a therapeutic use in human medicine(Reference Berni Canani, Di Costanzo and Leone57). Butyrate is part of a class of epigenetic factors known as HDAC inhibitors, with several functions such as anti-inflammatory and anti-carcinogenesis effects. Given the role of bacterial species in the production of SCFA, probiotics may be considered as an alternative approach for the prevention or treatment of chronic inflammatory diseases(Reference Licciardi, Wong and Tang53). Intestinal microflora recently also has been implicated in the development of some metabolic phenotypes, such as obesity and insulin resistance(Reference Dumas, Barton and Toye58). A longitudinal, prospective study in childhood revealed that a combination of early exposures, including delivery mode (vaginal v. by caesarean section), maternal pre-pregnancy BMI and antibiotics in infancy (less than 6 months of age), influences the risk of overweight in childhood, evaluated with a follow-up at age 7 years. This effect may potentially be explained by an impact on establishment and diversity of the microbiota(Reference Ajslev, Andersen and Gamborg59). Recently, Wang et al. (Reference Wang, Klipfell and Bennett60) identified a new direct link between gut flora-dependent metabolism of dietary phosphatidylcholine and CVD pathogenesis, which represents the leading cause of death and morbidity worldwide. These results indicate that an appropriately designed probiotic intervention may serve as a therapeutic strategy for the prevention and treatment of atherosclerotic heart disease and its complications(Reference Wang, Klipfell and Bennett60). In this regard, it will be important to evaluate in animal models of postnatal dietary manipulation the effects of specific nutrients on methylation and histone modifications, considering that some of the benefits attributed to breast milk may partly be due to the establishment of a beneficial bacterial flora in the gut of milk-fed infants, whichever the origin(Reference Poroyko, White and Wang61).

Fig. 1 The potential role of maternal diet on the development of the metabolic syndrome. Different epigenetic mechanisms may be involved in the long-lasting effects elicited by dietary factors in the development of the main components of the metabolic syndrome: diabetes, obesity, hypertension, dyslipidaemia and CVD.
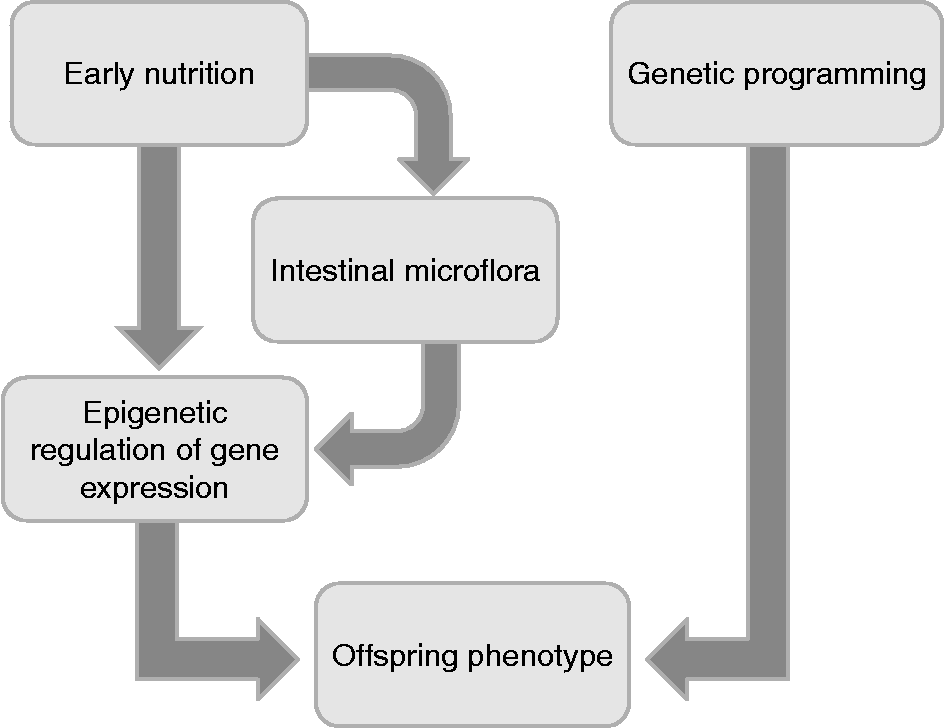
Fig. 2 Offspring phenotype is influenced by genetic programming and by epigenetic regulation of gene expression, elicited by prenatal/neonatal nutrition and intestinal microflora composition in early life.
Conclusions
The studies reviewed here suggest that maternal and neonatal diet may have long-lasting effects in the development of non-communicable chronic adulthood diseases, in particular the components of the so-called metabolic syndrome, such as insulin resistance, type 2 diabetes, obesity, dyslipidaemia, hypertension and CVD. Different epigenetic mechanisms may, at least in part, explain the way by which dietary factors in early critical developmental steps might be able to affect the susceptibility to develop metabolic diseases in adulthood. Both maternal under- and over-nutrition may interact with genes controlling lipid and carbohydrate metabolism, thus inducing alterations in epigenetic regulations. Early postnatal environment, including nutrition, may represent a vital determinant of adult health by making an impact on the development and function of gut microbiota. The epigenetic mechanisms elicited by probiotics through the production of SCFA are hypothesised to be the key to understand how they mediate their numerous health-promoting effects from the gut to overall health status. Much of the recent progress in understanding epigenetic phenomena is directly attributable to technologies that allow researchers to pinpoint the genomic location of proteins that package and regulate access to DNA. However, despite recent advances, our knowledge regarding nutritional epigenetics in early life is still limited. Further studies in human subjects using the latest technologies are needed to better understand the use of nutrients or bioactive food components for maintaining our health and preventing diseases through modifiable epigenetic mechanisms. Moreover, given the plasticity of epigenetic marks and their responsiveness to dietary factors, there is potential for the development of epigenetic marks as biomarkers of health for use in intervention studies.
Acknowledgements
B. C. R., D. C. M., L. L., B. G., B. P., C. S., N. V., P. A. and A. C. were responsible for the conceptualisation and implementation of the manuscript. B. C. R., D. C. M. and L. L. were responsible for writing the manuscript. All authors reviewed the manuscript and approved it before submission.
This review paper received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
There are no conflicts of interest.





