Introduction
Two types of buckwheat are used globally: common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (F. tataricum Gaertner). Buckwheat groats and flour have been established as nutritional foods because of their high levels of proteins, rutin, quercetin and minerals( Reference Bonafaccia, Marocchini and Kreft 1 , Reference Pongrac, Vogel-Mikus and Jeromel 2 ), such as Se( Reference Kreft, Mechora and Germ 3 ). In Europe, buckwheat bread is gaining significance due to its nutritional properties, antioxidant capacity and the possibility of preparing gluten-free bread( Reference Costantini, Lukšič and Molinari 4 ). Recently, buckwheat herb was suggested as a functional food. Milled dried plants may be added as colorant to pasta and other products( Reference Kim, Soon and Hwang 5 ). There are numerous reports of potential health benefits of consuming buckwheat, which may be in a form of food, dietary supplements, home remedies or possibly pharmaceutical drugs. Safety of any food and drugs is of great importance. Recently, a report of severe adverse effect of taking buckwheat tablets was published( Reference Yang, Yu and Wang 6 ). The authors reported five cases of new-onset polyneuropathy with dyskinesia induced by composite tablets of black tea and Tartary buckwheat used as a hypoglycaemic food supplement. The diagnosed polyneuropathy was relatively rare but severe; for this the present review of known potential health effects of buckwheat products is instrumental to assess the safety of using buckwheat products. First, it is important to note that the medical history of the affected patients revealed that all took tablets from the same batch( Reference Yang, Yu and Wang 6 ). This makes a strong assumption that contamination may have been the cause of reported acute symptoms, which developed quickly after taking this drug and ceased quickly after withdrawing from taking the tablets. The majority of patients had numbness and weakness of the limbs, paraesthesias, hoarseness and bladder dysfunction; one had either shortness of breath, dysphagia or facial paralysis. No heavy metal or other toxic contaminants were found in the tablet. This may indicate that some highly toxic contaminants present in low quantities were missed by the analyses( Reference Yang, Yu and Wang 6 ).
The present review addresses known potential health-related effects of buckwheat products. This topic is especially important in view of recent increased public interest in buckwheat (Fig. 1).
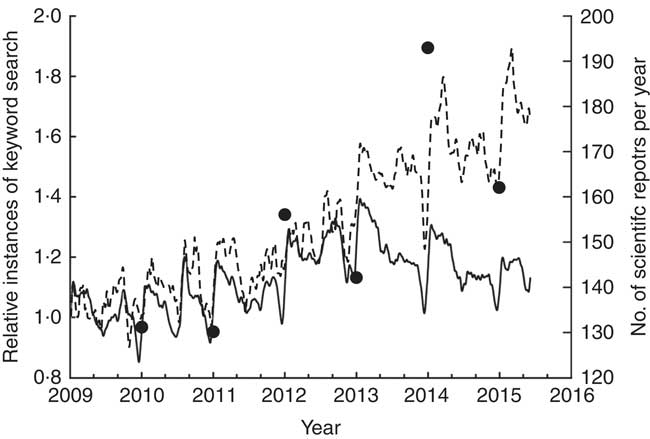
Fig. 1 Increase in public interest in buckwheat. Relative instances of the keyword ‘buckwheat’ (---) have increased in the last 3 years compared with the keyword ‘wheat’ (–––). Data were taken from Google Trends, which provides an index of the relative volume of search queries conducted through Google. Similarly, an increase in the number of publications on buckwheat (∙), indexed in the Web of Science (Thomson Reuters), is noticeable.
Adverse effects as a result of contamination of herbal medicinal products
The safety and quality of medicinal plant materials and herbal medicinal products are a major concern for health authorities and the public( 7 ). However, numerous adverse effects have been found as a result of adulteration or contamination of herbal medicinal products, such as agranulocytosis, meningitis, organ failure, perinatal stroke and heavy metal poisoning( Reference Posadzki, Watson and Ernst 8 ). Reports include neurological adverse effects such as paraesthesia and seizures( Reference Ernst 9 ). Unfortunately, the data are largely anecdotal. Plant products may be susceptible to attack by pathogenic, often mycotoxigenic, fungi with consequent increase of mycotoxins. Aspergillus flavus may produce aflatoxin B1 (AFB1), the most carcinogenic compound of fungal origin. Seeds of F. tataricum are less susceptible to A. flavus infection compared with F. esculentum ( Reference Chitarrini, Nobili and Pinzari 10 ).
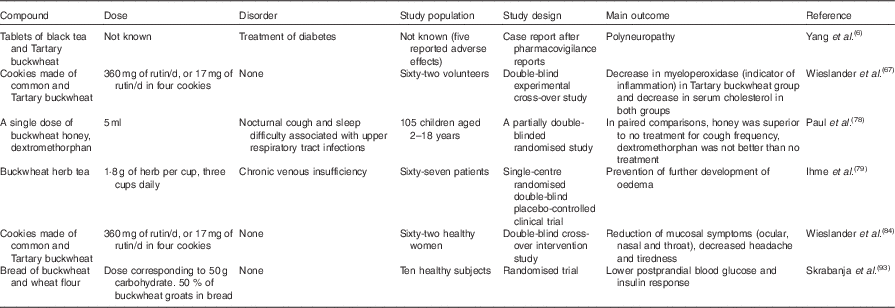
Neuropathy described after taking buckwheat tablets( Reference Yang, Yu and Wang 6 ) may be attributed to contamination with other herbs such as Psychotria rubra ( Reference Mak, Leung and But 11 ). Another example of a neurotoxic plant is hemlock (Conium maculatum), which contain piperidine alkaloids, and was probably the cause of death of Socrates( Reference Reynolds 12 ). Plants from the genus Senecio (Compositae), which contain hepatotoxic pyrrolizidine alkaloids, are a common cause of poisoning with herbal products( Reference Prakash, Pereira and Reilly 13 ). Chronic toxicity was found in daily doses of pyrrolizidine alkaloids as low as 25 μg( Reference Rasenack, Müller and Kleinschmidt 14 ). Botulinum toxins from the bacterium Clostridium botulinum are the most potent toxins, with the lethal dose for an individual by the oral route being 30 ng( Reference Peck 15 ). Clostridia should be absent in all herbal materials, preparations and finished herbal products, as recommended by Annex 5 of WHO guidelines( 7 ). Foods that are fermented and consumed without cooking pose a substantial risk: first, because Clostridia are obligate anaerobes; second, the toxin is degraded by cooking( Reference Sobel 16 ). The clinical syndrome of botulism includes symmetrical cranial nerve palsies and flaccid paralysis. This may manifest as an expressionless face, dysphagia, dry mouth, shortness of breath( Reference Sobel 16 , Reference Varma, Katsitadze and Moiscrafishvili 17 ) and paraesthesia( Reference Hughes, Blumenthal and Merson 18 ). The signs of botulism resemble some signs described in poisoning with buckwheat product( Reference Yang, Yu and Wang 6 ). Clostridium spp. bacteria have been previously discovered in traditional Chinese herbal medicines, such as Xiyangshen root and Dangshen root( Reference Ting, Chow and Tan 19 ). Other than possible contaminants, adverse effects may also be due to plant metabolites, naturally present in food and plant products. Here the current literature to elucidate if there is any previous indication that peripheral nerve damage could appear in patients taking buckwheat is reviewed( Reference Yang, Yu and Wang 6 ). Bibliographic data, analysed in the year 2015, are summarised in Tables 1, 2 and 3.
Table 1 In vitro tests of buckwheat activity and activity of its metabolites

DPPH, 1,1-diphenyl-2-picrylhydrazyl; TEAC, Trolox equivalent antioxidant capacity; ORAC, oxygen radical absorbance capacity; ABTS, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); EC50, half maximal effective concentration.
Table 2 Main published preclinical trials dealing with the effect of buckwheat and its metabolites on experimental animals

ICR, Imprinting Control Region.
Table 3 Main published clinical trials dealing with the effect of buckwheat consumption on human health
Phenolic metabolites in buckwheat
Buckwheat is mainly grown for the production of seeds( Reference Wijngaard and Arendt 20 ). It is an important functional food, rich in vitamins, essential amino acids and phenolic compounds( Reference Holasova, Fiedlerova and Smrcinova 21 ). The content of rutin in Tartary buckwheat herb is as high as 3 % dry weight, and up to 1·7 % in seeds( Reference Fabjan, Rode and Kosir 22 ). In common buckwheat milling products rutin content is two orders of magnitude less than in Tartary buckwheat seeds and is highly variable (from 19 to 160 mg/kg in different flour fractions and 480 mg/kg in bran)( Reference Kreft, Knapp and Kreft 23 ). In milling fractions darker colour was also correlated with higher protein and minerals content( Reference Skrabanja, Kreft and Golob 24 ). This variability indicates that rutin and nutrients are not equally distributed in the seed, and is attributed to specific seed morphology( Reference Kreft and Kreft 25 , Reference Kreft and Kreft 26 ).
Therapeutic doses of rutin have been estimated to be between 180 and 350 mg( Reference Schilcher, Patz and Schimmel 27 ). Thus, the daily intake of 100 g of buckwheat flour or bran in food would cover 10 % of the therapeutic dose. This contributes to average doses of flavonols and flavons otherwise consumed by at least 2-fold( Reference Hertog, Holman and Katan 28 ).
The composition and differential content of phenolic compounds in seeds of common buckwheat were recently analysed( Reference Kiprovski, Mikulic-Petkovsek and Slatnar 29 ). The list of flavonoids, including rutin, is shown in Table 4. The most detected hydroxycinnamic acids in seeds are caffeic and chlorogenic acid derivatives( Reference Kiprovski, Mikulic-Petkovsek and Slatnar 29 ).
Table 4 Flavonoids from common buckwheat seeds( Reference Kiprovski, Mikulic-Petkovsek and Slatnar 29 )

From Tartary buckwheat (F. tataricum) grains a preparative separation successfully purified five flavonoids: quercetin, kaempferol, quercetin 3-O-rutinoside-3′-O-β-glucopyranoside, rutin and kaempferol 3-rutinoside( Reference Jiang, Liu and Xie 30 ) (Fig. 2). Flavonoid metabolism is related to responses to UVB radiation( Reference Regvar, Bukovnik and Likar 31 ). Recently, a new Tartary buckwheat cultivar, ‘Manten-Kirari’, has been developed, whose grains contain only trace amounts of rutinosidase and lack bitterness. This is a promising variety for preparing non-bitter, rutin-rich foods( Reference Suzuki, Morishita and Mukasa 32 ). The bread-baking procedure using Tartary buckwheat has an impact on rutin, quercetin and polyphenol concentration and antioxidant activity. Rutin concentration during the bread-baking process decreases, while the concentration of quercetin remains stable( Reference Vogrincic, Timoracka and Melichacova 33 ). Similarly, there is much less rutin in noodles compared with flour made from buckwheat( Reference Kreft, Fabjan and Yasumoto 34 ).
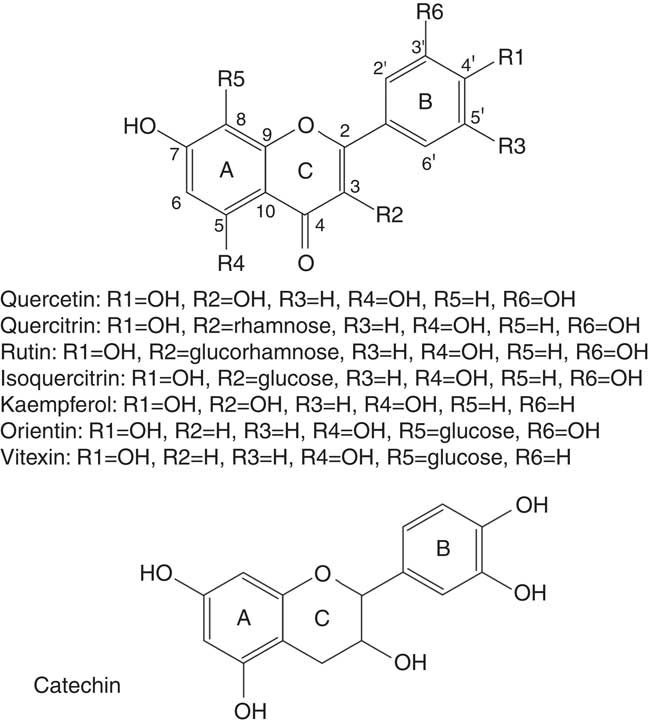
Fig. 2 Structures of main flavonoids found in buckwheat. Flavones have A, C and B ring structures, with substitutions as indicated at B4′ (R1), C3 (R2), B5′ (R3), A5 (R4), A8 (R5) and B3′ (R6).
A much higher rutin level than in seeds is found in fresh buckwheat shoots, which are consumed as a salad or cooked( Reference Kreft, Chang and Choi 35 ). The buckwheat plant has the highest concentration of rutin and epicatechin in the leaves and flowers( Reference Kalinova, Triska and Vrchotova 36 ), depending on UV irradiation( Reference Kreft, Strukelj and Gaberscik 37 ). Interestingly, shoots grown from seeds soaked in selenite or selenate solution had higher total flavonoids content compared with soaking seeds in water( Reference Ozbolt, Kreft and Kreft 38 ). Additionally to flavonoids, found in seeds, common buckwheat sprouts also contain vitexin, isovitexin and quercetin-3-O-robinobioside( Reference Nam, Lee and Park 39 ).
It is important to note that before absorption, dietary phenolic compounds may be transformed in the small intestine by digestive enzymes and in the colon by the intestinal microbiota system( Reference Selma, Espín and Tomás-Barberán 40 ). For example, rutin may be converted to quercetin, depending on its concentration and composition of the gut microflora( Reference Rechner, Smith and Kuhnle 41 ). Although quercetin is metabolised preferentially to carbon dioxide, the biological half-life is very long, ranging from 20 to 72 h( Reference Walle, Walle and Halushka 42 ). Furthermore, the absorption of quercetin taken orally is surprisingly high, ranging from 36 to 53 %( Reference Walle, Walle and Halushka 42 ), but relatively slow, since it takes 6 h for the plasma concentration to steadily reach the peak concentration( Reference Murota, Matsuda and Kashino 43 , Reference Erlund, Kosonen and Alfthan 44 ).
Other metabolites of buckwheat
In addition, buckwheat sprouts contain naphthodianthrone fagopyrins that can cause photosensitisation( Reference Benkovic, Zigon and Friedrich 45 , Reference Tavčar Benković and Kreft 46 ), manifested as skin irritation after sunlight exposure. The highest levels of fagopyrins have been found in flowers and leaves( Reference Stojilkovski, Glavac and Kreft 47 ). Levels of fagopyrins increase gradually in sprout growth, especially in the light( Reference Kreft, Janeš and Kreft 48 ).
Products prepared from common buckwheat grains have a characteristic aroma, which is attributed mainly to salicylaldehyde( Reference Janeš and Kreft 49 ). Other significant odorants are (E,E)-2,4-decadienal, (E)-2-nonenal, 2-pheniletanol, (E,E)-2,4-nonadienal, hexanal, decanal, nonanal, and, in Tartary buckwheat, naphthalene( Reference Janeš, Prosen and Kreft 50 – Reference Janeš, Prosen and Kreft 53 ). The pharmacological and medical effects of these volatile compounds need to be further investigated.
Antioxidant activity of buckwheat
Experiments using an in vitro human digestion model showed that the antioxidative activity of common buckwheat is increased by digestion in the small intestine via an increase in the antioxidants rutin and quercetin( Reference Hur, Park and Jeong 54 ). The antioxidant capacity of quercetin from Tartary buckwheat was the strongest, compared with isoquercetin and rutin( Reference Li, Duan and Jia 55 ). On the other hand, by reducing the intracellular antioxidase activity, flavonoid compounds could increase cell oxidative stress( Reference Fang, Nakamura and Iyer 56 ). It has been shown that quercetin exhibits the strongest cytotoxic effects against the human hepatoma cell line, which is due to the G2/M phase arrest accompanied by an increase of apoptotic cell death. The p53 and p21 were found to be up-regulated, and cyclin D1, Cdk2 and Cdk7 down-regulated( Reference Li, Duan and Jia 55 ). Further, it has been shown that in the human hepatoma cell line, common and Tartary buckwheat has antigenotoxic effects( Reference Vogrinčič, Kreft and Filipič 57 ). The potential anti-tumour effect of flavonoids of buckwheat has not yet been thoroughly studied. An epidemiological study on 738 men showed that intake of flavonoids does not predict a reduced risk of cancer in elderly men( Reference Hertog, Feskens and Hollman 58 ).
With germination of buckwheat seeds, phenolic compounds, such as rutin, vitexin, isovitexin, orientin, isoorientin, chlorogenic acid, trans-3-hydroxycinnamic acid and p-hydroxybenzoic acid increased significantly, which may be due to the activation of phenylalanine ammonialyase( Reference Zhang, Xu and Gao 59 ). This leads to significant enhancement of the antioxidant activities of germinated buckwheat and may be used as a promising functional food( Reference Zhang, Xu and Gao 59 ). Buckwheat sprouts are suggested as a new vegetable( Reference Kim, Kim and Park 60 ). The highest total phenols in buckwheat sprouts of germinated soaked buckwheat seeds is at day 6( Reference Koyama, Nakamura and Nakamura 61 ) or day 8( Reference Lin, Peng and Yang 62 ). Specifically, compared with buckwheat seeds, the sprouts contain relatively large amounts of rutin( Reference Kreft, Janeš and Kreft 48 , Reference Kim, Kim and Park 60 ).
Common buckwheat and Tartary buckwheat sprouts have different antioxidant activities. It has been shown that the ethanol extracts of Tartary buckwheat sprouts have higher reducing power, free radical scavenging activity, and superoxide anion scavenging activity than those of common buckwheat sprouts, possibly because of their higher rutin and quercetin content( Reference Lin, Peng and Yang 62 ). Treatment with raw common buckwheat extract and germinated buckwheat extract reduced oxidative damage in aortic endothelial cells by lowering nitrotyrosine immunoreactivity, which suggests an antihypertensive effect and may protect arterial endothelial cells from oxidative stress( Reference Kim, Hwang and Lim 63 ). In Tartary buckwheat, the compounds which play a key role in the elevated antioxidant activities during germination consisted of vitamin C, total flavonoids and rutin, but not vitamin E and quercetin( Reference Zhou, Hao and Zhou 64 ).
CVD, hypertension and plasma cholesterol
Food rich in polyphenols possess cardiovascular protective properties( Reference Andriantsitohaina, Auger and Chataigneau 65 ), and antihypertensive properties( Reference Rodrigo, Gil and Miranda-Merchak 66 ). Specifically, buckwheat products reduce the serum levels of myeloperoxidase and cholesterol( Reference Wieslander, Fabjan and Vogrincic 67 ). The reduction of serum cholesterol by common and Tartary buckwheat protein products is associated with enhanced excretion of faecal neutral sterols bile acids in mice and rats( Reference Tomotake, Yamamoto and Kitabayashi 68 ). Extract of germinating common buckwheat seeds, administered orally to mice, reduces hepatic TAG and total cholesterol, and down-regulates the expression of adipogenic transcription factors PPARγ and C/EBPα in hepatocytes( Reference Choi, Seog and Park 69 ). Some earlier studies indicated reduced senile hyperlipidaemia, reduced blood pressure and reduction of weight; however, these trials were without control groups (for a review, see Wieslander & Norbäck( Reference Wieslander and Norbäck 70 )).
It has been shown that spontaneously hypertensive rats fed Tartary buckwheat sprouts exhibit higher plasma levels of the endogenous vasodilators bradykinin and NO, a lower level of the vasoconstrictor endothelin-1, and an improved antioxidant capacity, which may collectively reduce hypertension and oxidative stress in vivo ( Reference Merendino, Molinari and Costantini 71 ). A potent vasorelaxant effect was found in the (+)-osbeckic acid dimer, which was isolated from rutin-free Tartary buckwheat extract( Reference Matsui, Kudo and Tokuda 72 ). Tartary buckwheat rutin-free extracts exert endothelium-dependent vasorelaxation action in isolated rat aorta rings, probably by NO/cGMP signalling pathways( Reference Ushida, Matsui and Tanaka 73 ). Buckwheat rutin exhibits an inhibitory effect on angiotensin II-induced hypertrophy in cultured neonatal rat cardiomyocytes via Ca2+ antagonism action, thus blocking the calcineurin-dependent signal pathway( Reference Chu, Li and Gao 74 ). Tartary buckwheat protein and not rutin exhibit angiotensin I-converting enzyme inhibition. Oral administration of Tartary buckwheat digest has been found to lower the blood pressure of hypertensive rats( Reference Li, Matsui and Matsumoto 75 ).
Immune system and inflammation
Dietary supplementation with buckwheat flour appears to have a protective effect on immune cell functions in mice with premature senescence( Reference Alvarez, Alvarado and Puerto 76 ). Several parameters of innate immune response were increased: macrophage chemotaxis, phagocytosis, microbicidal activity, natural killer activity, as well as parameters of acquired immune response: lymphoproliferative response to concanavalin A and lipopolysaccharide, and IL-2 release( Reference Alvarez, Alvarado and Puerto 76 ).
Flavonoids including quercetin have shown viral inhibition properties such as antiherpetic activity against Herpes simplex virus, types 1 and 2( Reference Garrett, Romanos and Borges 77 ). A survey on parents of 105 children with upper respiratory tract infections was performed to compare the effects of a single dose of buckwheat honey or honey-flavoured dextromethorphan with no treatment. Significant differences in symptom improvement were detected between treatment groups, with honey consistently scoring the best and no treatment scoring the worst( Reference Paul, Beiler and McMonagle 78 ). However, it was not yet established if honey in general or buckwheat honey specifically was favourable for the relief of coughing.
Ihme et al. ( Reference Ihme, Kiesewetter and Jung 79 ) investigated the effect of buckwheat herb tea in treating leg oedema in patients with chronic venous insufficiency. Results of a randomised double-blind placebo-controlled clinical trial indicated potential use in patients to prevent the further development of oedema. As importantly, the study on sixty-seven patients confirmed the safety of this treatment( Reference Ihme, Kiesewetter and Jung 79 ).
Rutin has potential anti-inflammatory properties( Reference Ku, Lee and Han 80 ). It is a potent inhibitor of phorbol-12-myristate 13-acetate (PMA), TNF-α, IL-1β, and caecal ligation and puncture (CLP)-mediated endothelial cell protein C receptor shedding( Reference Ku, Lee and Han 80 ). Extract of buckwheat sprouts was shown to inhibit pro-inflammatory mediators IL-6 and TNF-α production in macrophages( Reference Karki, Park and Kim 81 ). Extracts from Tartary buckwheat were shown to exert hepatoprotection via promoting antioxidative and anti-inflammatory properties against oxidative liver damage in mice. This was manifested as inhibiting the increase in serum aspartate transaminase, alanine transaminase and alkaline phosphatase levels in challenged animals( Reference Lee, Shen and Lai 82 ). A buckwheat protein diet may retard the development of mammary tumours in female rats, which was found to be correlated with lower serum oestradiol( Reference Kayashita, Shimaoka and Nakajoh 83 ).
A double-blind cross-over intervention study was conducted to study the effects of common and Tartary buckwheat consumption on mucosal symptoms, i.e. ocular, nasal and throat symptoms; further, headache and tiredness were evaluated( Reference Wieslander, Fabjan and Vogrincic 84 ). Both types of buckwheat had generally positive effects on these symptoms.
Neurological disorders
It was recently shown that the n-butanol fraction and rutin extracted from Tartary buckwheat are protective against and have possible therapeutic applications for the treatment of Alzheimer’s disease( Reference Choi, Lee and Lee 85 ). This was confirmed by studying learning and memory deficits in a mouse model of amyloid β-induced Alzheimer’s disease. Animals’ impaired cognition and memory were alleviated by the oral administration of an n-butanol fraction and rutin extracted from Tartary buckwheat( Reference Choi, Lee and Lee 85 ).
Rutin’s protective effects against acetaldehyde-based ethanol neurotoxicity have been found. Rutin protects hippocampal neuronal cells against ethanol-induced neurotoxicity by increasing aldehyde dehydrogenase 2 (ALDH2) activity. Its metabolite, acetaldehyde, is critically toxic. ALDH2 metabolises acetaldehyde into non-toxic acetate( Reference Song, Kim and Na 86 ). Rutin was suggested as a protective compound against the haloperidol-induced motor disorder orofacial dyskinesia, resulting from the chronic neuroleptic treatment of schizophrenia( Reference Bishnoi, Chopra and Kulkarni 87 ). Haloperidol induces oxidative damage in all regions of the brain in rats, which was prevented by rutin, which may be a possible therapeutic to treat this motor disorder( Reference Bishnoi, Chopra and Kulkarni 87 ).
Weight regulation and diabetes
Tartary buckwheat is used for the treatment of type 2 diabetes mellitus in Taiwan. It has been shown that the ethanol extract of buckwheat and rutin attenuates protein glycation to lower the generation of advanced glycation endproducts through the suppression of fructosamine and α-dicarbonyl compounds; hence it may be used as a protection agent in diabetic patients( Reference Lee, Lee and Lai 88 ). The ethanol extract of buckwheat, rutin and quercetin improved glucose uptake via promoting Akt phosphorylation and preventing PPARγ degradation in a hepatocyte cell line( Reference Lee, Hsu and Shen 89 ). Buckwheat bran extracts and not pure rutin inhibit sucrase activity in vitro, which may have a beneficial effect on diabetes( Reference Hosaka, Nii and Tomotake 90 ). Similarly, it seems that buckwheat concentrate has insulin-mimetic effects on select protein phosphorylation events in rat hepatoma cells; however, d-chiro-inositol and myo-inositol are not probably active components responsible for the observed effects( Reference Curran, Stringer and Wright 91 ). Rutin was found to attenuate the induced glucotoxicity in β-cells by stimulating insulin receptor substrate 2 signalling in rat pancreatic β-cells. The intrinsic protective effects of rutin in preserving insulin signalling may lead to novel strategies for the prevention of type 2 diabetes( Reference Cai and Lin 92 ).
In healthy subjects consuming bread with buckwheat and wheat flour, lower postprandial blood glucose and insulin response were measured, compared with a group eating wheat bread( Reference Skrabanja, Liljeberg Elmståhl and Kreft 93 ). Proanthocyanidins in buckwheat flour can reduce salivary nitrite to NO in the stomach. This may improve the activity of the stomach, helping the digestion of ingested foods( Reference Takahama, Tanaka and Hirota 94 ). Proanthocyanidins from persimmon inhibit oxidative stress and the digestive enzymes related to diabetes, such as α-amylase and α-glucosidase( Reference Lee, Cho and Tanaka 95 ).
Buckwheat leaf and flower food supplementation apparently reduces weight gain, plasma lipid concentrations and atherogenic index in rats fed a high-fat diet; buckwheat products are thus suggested for the potential prevention and curing of hyperlipidaemia( Reference Ðurendić-Brenesel, Popović and Pilija 96 ).
Summary
Numerous reports have shown the potential health benefits of consuming buckwheat, which may be in the form of food, dietary supplements, home remedies or possibly pharmaceutical drugs. There are reports of the antioxidative activity of buckwheat; on the other hand, both cytotoxic and antigenotoxic effects have been shown. Reduction of hyperlipidaemia, reduction of blood pressure and improved weight regulation have been suggested. Consuming buckwheat may have beneficial effect on diabetes. Rutin was also suggested to have potential therapeutic applications for the treatment of Alzheimer’s disease. It can be concluded that the literature indicates that buckwheat is safe to consume and may have various beneficial effects on human health.
Acknowledgements
Valuable comments and the help of Professor Ivan Kreft (Biotechnical Faculty, University of Ljubljana & Slovenian Forestry Institute) and Professor Samo Kreft (Faculty of Pharmacy, University of Ljubljana) are gratefully acknowledged.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
There are no conflicts of interest.









