Introduction
Systemic lupus erythematosus (SLE) can be defined as a chronic inflammatory and autoimmune disease that can affect multiple organ systems, including skin, joints, kidneys and the brain, among others( Reference Noble, Bernatsky and Clarke 1 ). The clinical heterogeneity of this remarkable and challenging disorder has required the establishment of eleven criteria with four needed for the formal diagnosis of SLE( Reference Tan, Cohen and Fries 2 ). The action of pathogenic factors results in the generation of autoantibodies, immune complexes, autoreactive or inflammatory T cells and inflammatory cytokines that may initiate and amplify inflammation and damage to various organs, contributing to the clinical manifestations of SLE. Moreover, heritable, hormonal and environmental factors contribute to the expression of organ damage. SLE is characterised by a deposition of immune complexes, formed in large amounts as antinuclear antibodies bind to the abundant nuclear material in blood and tissues, along with disturbances in both innate and adaptive immunity manifest by disorders in cytokines, apoptotic cell clearance, B-cell immunity and T-cell signalling( Reference Crispín, Liossis and Kis-Toth 3 , Reference Lisnevskaia, Murphy and Isenberg 4 ).
It is also characterised by its clinical and pathogenic complexity, difficult diagnosis and the high number of complications that can affect the patients’ quality of life. The high morbidity and mortality associated with patients with SLE may be related to late diagnosis, problems in access to care, less effective treatments and poor adherence to therapeutic regimens( Reference Petri, Orbai and Alarcón 5 ).
SLE incidence has been estimated to range between 1 and 10 cases per 100 000 individuals per year, and the prevalence has been reported to range between 20 and 150 cases per 100 000 individuals worldwide with large regional and ethnic variations( Reference Vina, Utset and Hannon 6 ). SLE is a disease that can affect both sexes, but more than 90 % of new patients presenting with SLE are women in the childbearing years. In addition, although the disease may begin at any age, it appears most often at the end of the patients’ second decade of life and at the beginning of the third decade( Reference Mina and Brunner 7 ). SLE affects multiple systems and its presentation and course are highly variable, ranging from indolent to fulminant. The most common clinical manifestations include fatigue, loss of appetite and weight, cutaneous lesions (mainly malar rash), arthritis, serositis (pleuritis and/or pericarditis), renal or central nervous system involvement and haematological manifestations (cytopenias) associated with several autoantibodies, particularly antinuclear antibodies( Reference Petri, Orbai and Alarcón 5 ).
Additionally, patients with SLE show an increased risk of atherosclerosis and vascular events, which contribute to increase the morbidity and mortality of these patients. In SLE, autoantibodies and cytokines are able to modulate and decrease lipoprotein lipase activity, a key enzyme in lipid metabolism, producing the ‘lupus pattern’ of dyslipoproteinaemia characterised by elevated levels of VLDL and TAG and low HDL-cholesterol levels, which are directly correlated with SLE disease activity index (SLEDAI) scores( Reference Borba, Carvalho and Bonfá 8 ). The mechanisms underlying this enhanced risk are still not clear, but some theories include excess of monocyte activation, dysregulation of the complement system, oxidative stress and production of different antibodies to endothelial cells, anti-atherogenic HDL, anti-lipoprotein lipase and oxidised LDL among others( Reference Lisnevskaia, Murphy and Isenberg 4 ).
Systemic lupus erythematosus aetiology
The aetiology of SLE is not fully known. Genetics is thought to play a crucial role in the development of SLE, and multiple genes that contribute to the predisposition and susceptibility to SLE have been identified, including IRF5, STAT4, osteopontin, IRAK1, TREX1 and TLR8, and genes related to interferon (IFN) production or implicated in the signalling mechanisms mediated by T (PTPN22, TNFSF4, PDCD1) or B (BANK1, BLK, LYN) cells( Reference Moser, Kelly and Lessard 9 ). In addition, recent studies have shown that several regions located in the major histocompatibility complex as well as BsmI and FokI polymorphisms contribute to increased SLE risk( Reference Xiong, He and Zeng 10 , Reference Ruiz-Larrañaga, Migliorini and Uribarri 11 ). Environmental factors, including extreme stress, exposure to UV light, smoking, infections, administration of certain drugs (some antidepressants and antibiotics) and hormonal factors (oestrogen) can also contribute to the disease progression( Reference Mirabelli, Cannarile and Bruni 12 – Reference Mak and Tay 14 ). Nevertheless, over recent years, multiple lines of evidence have supported a major role of specific dietary factors, including vitamins, mineral elements, fatty acids and polyphenols, in the modulation of immune responses. Thus, an inadequate diet could constitute an important risk factor in SLE epidemiology( Reference Selmi 15 – Reference Rodríguez Huerta, Trujillo-Martín and Rúa-Figueroa 18 ).
The pathological hallmark of SLE is altered immune response with loss of B and T lymphocyte self-tolerance resulting in hyperactivity, autoantibody production (antinuclear antibodies, anti-double-stranded DNA (anti-dsDNA), anti-Ro, anti-histone, anti-Smith, anti-ribonucleoprotein and anti-ribosomal P protein, among others), aberrant immune complex formation and generation of a systemic inflammatory response in which multiple organs are involved: kidneys, heart, joints, skin, lungs, blood vessels, liver and nervous system( Reference Podolska, Biermann and Maueröder 19 ). The course of the disease is unpredictable, with periods of exacerbations and remissions( Reference Crispín, Liossis and Kis-Toth 3 ). Autoantibodies, immune complexes and an imbalance of T-helper-cell subsets (Th1/Th2/Th17) and regulatory T-cells (Tregs) contribute to tissue damage and could be responsible for an increased proinflammatory response, especially in the active form of the disease( Reference Dolff, Bijl and Huitema 20 ). In particular, patients with active SLE exhibit high cytokine levels, including IFN-γ, TNF, IL-4, IL-6, IL-10, IL-12, IL-17 and IL-18 in serum and plasma; by contrast, IL-2 levels are lower in comparison with healthy controls( Reference Chun, Chung and Kim 21 ). In addition, recent research has associated SLE development with changes in the dendritic cell compartment( Reference Ganguly, Haak and Sisirak 22 ). Likewise, it has been suggested that γδ T cells may be involved in the regulation of SLE owing to their functions in cytokine secretion, antigen presentation and supporting B cells in antibody production( Reference Wu, Yang and Li 23 ). On the other hand, many studies have demonstrated quantitative and/or qualitative defects of Tregs (phenotype CD4+ CD25 high forkhead box P3 (FOXP3)+) in patients with SLE as well as a reduction in the number of natural killer (NK) cells. Moreover, an enhanced Th17 cell response correlates with disease activity in patients with SLE, suggesting a role for IL-17 in the pathogenesis of the disease owing to its ability to amplify local inflammation by recruiting innate immune system cells and to stimulate the adaptive immune response in conjunction with B-cell activating factor (BAFF)( Reference Nalbandian, Crispín and Tsokos 24 , Reference Gaffen 25 ). The molecular signalling pathways involved in SLE include NF-κB, FOXP3, mitogen-activated protein kinase (MAPK), Janus kinase and signal transducer and activator of transcription (JAK/STAT) and, more recently, NLR family pyrin domain-containing 3 (NLRP3) inflammasome and nuclear factor E2-related factor 2 (Nrf-2) pathways( Reference Nie, Li and Zheng 26 – Reference Zhao, Wang and Huang 30 ). The processes of adhesion, extravasation and subsequent activation of neutrophils in SLE are due to increases in the expression of intercellular adhesion molecule-1 (ICAM-1), endothelial adhesins, selectins and integrins. The secretion of proteases, reactive oxygen species and myeloperoxidase by the neutrophils then contributes to the cell damage( Reference Telles, Ferreira and da Silva 31 ). Finally, an increased apoptotic burden that determines the recognition of apoptotic-derived autoantigens and hyperactivation of innate and adaptive immune system cells has been described in human SLE and murine models of the disease( Reference Squatrito, Emmi and Silvestri 32 ).
Aim of systemic lupus erythematosus treatment
Nowadays, the overall aim of SLE therapy is to control disease activity. Nevertheless, SLE management remains complicated owing to the biological heterogeneity between patients and the lack of safe and specific targeted therapies. Thus, the search for new therapeutic targets and strategies that can act more selectively on certain routes or biological processes and improve the course of disease or reverse the outbreak phase without generating collateral damage to unaffected tissues and organs is the pillar underlying current research in SLE.
Typical SLE management includes the use of antimalarial drugs (mainly hydroxychloroquine and chloroquine), immunosuppressive agents, biological agents and some adjunctive therapies following international recommendations( Reference Kuhn, Bonsmann and Anders 33 ). Mild activity can be managed with non-steroidal anti-inflammatory drugs or low-dose glucocorticoids, but more severe manifestations require more advanced treatment( Reference Kuhn, Bonsmann and Anders 33 – Reference Bertsias, Tektonidou and Amoura 35 ). Glucocorticoids act as anti-inflammatory and immunosuppressive agents, but they are associated with several severe side effects including osteoporosis, cataracts, hyperglycaemia and cognitive impairment, among others( Reference Buttgereit, Saag and Cutolo 36 , Reference Ruiz-Irastorza, Danza and Khamashta 37 ). In particular, glucocorticoid therapy may increase the predisposition to obesity, leading to increased levels of proinflammatory cytokines that exacerbate the SLE symptoms and increase the risk of developing diabetes mellitus, hypertension and CHD symptoms. Moreover, the chronic use of corticosteroids in SLE is associated to an increase of total plasma cholesterol and its fractions (LDL and HDL) levels, and also TAG( Reference Borba, Carvalho and Bonfá 8 ). On the other hand, antimalarial drugs have shown beneficial effect on lipids and diabetes control in patients with SLE( Reference Gerstein, Thorpe and Taylor 38 – Reference Borba and Bonfá 40 ). If glucocorticoid administration cannot be reduced or discontinued, the administration of immunosuppressive disease-modifying anti-rheumatic drugs, commonly mycophenolate mofetil, cyclophosphamide, azathioprine, calcineurin inhibitors (particularly tacrolimus and cyclosporine A) and methotrexate, is recommended, with a tailored treatment regimen for every patient( Reference Bertsias, Ioannidis and Boletis 41 , Reference Hahn, McMahon and Wilkinson 42 ). However, the continuous use of immunosuppressive drugs may increase susceptibility to infections and gastrointestinal symptoms.
The increased knowledge about the aetiopathogenesis of SLE has enabled the use of biological agents specifically targeting the different pathways implicated in the disease( Reference Jordan, Lutalo and D’Cruz 43 ). Since B cell deregulation is one of the SLE hallmarks, B-cell-targeted therapies have become an important focus of SLE research. At present, belimumab, a monoclonal antibody that blocks BAFF, is the only approved biological drug for SLE, but other B-cell-targeted agents, such as rituximab, epratuzumab, blisibimod and tabalumab, are currently undergoing clinical evaluation, which has produced interesting results supporting their potential role in SLE treatment( Reference Hui-Yuen, Reddy and Taylor 44 – Reference Witcher, Fleischmann and Chindalore 49 ). Abatacept, which acts by blocking interactions between T and B cells, has shown efficacy in lupus mouse models, but controversial results have been described in human controlled trials( Reference Daikh and Wofsy 50 , Reference Furie, Nicholls and Cheng 51 ). Moreover, high serum levels of IFN in patients with SLE have been correlated with SLE disease activity. Recently, anti-IFN therapies, specifically sifalimumab, rontalizumab, anifrolumab and AMG 811, have been investigated with promising results, but several hurdles in their development must still be overcome and further studies are requested to fully determine their effects in SLE( Reference Mathian, Hie and Cohen-Aubart 52 ).
Thus, although pharmacological treatment for SLE has improved during the last decade, and many potential new agents are in development, SLE and its treatments contribute to increased mortality rates and the outcome remains unoptimistic in a considerable percentage of patients( Reference Postal, Sinicato and Appenzeller 53 ).
Diet therapy and nutrients in systemic lupus erythematosus
Nutritional therapy, including diet modification and the use of nutritional supplements, could be a promising way to approach SLE owing to both its potential prophylactic effects, without the side effects of the classic pharmacological therapy, and its possible contribution to reducing co-morbidities and improving the quality of life of patients with SLE( Reference Greco, Nakajima and Manzi 54 ).
Diet quality in patients with SLE is important since these patients, in addition to being at higher risk of CVD, are also at higher risk of low bone mineral density, high blood homocysteine levels and anaemia, which are directly influenced by diet( Reference Shah, Adams-Huet and Kavanaugh 55 ). It is important to highlight that more than half of the patients with SLE present three or more risk factors for CVD (mostly obesity, hypertension and dyslipidaemias), been certainly more susceptible to suffer with the metabolic syndrome( Reference Bruce 56 , Reference Chung, Avalos and Oeser 57 ). Moreover, obesity leads to increased levels of proinflammatory cytokines, which may exacerbate the inflammatory processes of SLE and increase the risk of diabetes mellitus, atherosclerosis and CHD( Reference Oeser, Chung and Asanuma 58 ). Additionally, a hyperlipidic diet, rich in cholesterol and saturated fat, is one of the major risk factors for maintaining dyslipidaemia in patients with SLE, perpetuating and aggravating lipid profile changes( Reference Klack, Bonfa and Borba Neto 59 ). Thus, we can suggest that the nutritional status and food intake of patients with SLE may interfere in the disease course and that an adequate diet may improve SLE prognosis and prevent several related diseases. Currently, a diet rich in vitamin- and mineral-rich foods and MUFA/PUFA with moderate energy consumption is recommended to control the inflammatory findings of the disease and the complications and co-morbidities resulting from SLE therapy( Reference Minami, Sasaki and Arai 60 , Reference Borges, dos Santos and Telles 61 ).
The restriction of energy in the diet has, in particular, shown beneficial effects in murine lupus models. An initial study revealed that a 40 % food-restricted maize oil-based diet delayed the onset of autoimmunity in New Zealand black/white (NZB/W) F1 lupus-prone mice( Reference Fernandes, Yunis and Good 62 ). Later studies demonstrated that energy restriction prevented the decline in CD8+ T lymphocytes, eliminated the abnormal increase in IL-12, IFN-γ, IgA and IgG2 production, delayed the development of kidney disease and age-related immune dysfunction and prolonged the lifespan in aged NZB/W F1 mice, probably owing to the down-regulation of mRNA expression or NF-κB( Reference Jolly, Muthukumar and Avula 63 , Reference Muthukumar, Jolly and Zaman 64 ).
In this regard, there is evidence that dietary factors can contribute to the geoepidemiology of autoimmune diseases( Reference Selmi 15 ). Recent studies have suggested that the traditional Mediterranean diet might confer protection from certain chronic diseases related to oxidative stress, inflammation and the immune system. This diet emphasises the intake of vegetables, fruits, nuts, grains and fish, along with small amounts of wine and olive oil as the main monounsaturated fat source, and limits meat consumption. The beneficial effects of the Mediterranean diet have been proven in cancer, CVD, obesity and arthritis( Reference Cárdeno, Sánchez-Hidalgo and Alarcón-de-la-Lastra 65 – Reference Casas, Sacanella and Estruch 67 ). Likewise, epidemiological data have shown a lower prevalence of rheumatic diseases in Mediterranean countries when compared with Northern Europe, and some clinical trials have demonstrated that the Mediterranean diet improves rheumatic symptoms and decreases the use of anti-inflammatory drugs and classic pharmacotherapy-related side effects( Reference Miggiano and Gagliardi 68 , Reference McKellar, Morrison and McEntegart 69 ).
The broad range of evidence demonstrating the antioxidant, anti-inflammatory and immunomodulatory effects of some nutrients in immunoinflammatory diseases has suggested a possible supportive role for these nutrients in the primary and secondary prevention of SLE.
However, the question arises as to whether different nutrients could ameliorate or exacerbate SLE symptoms and how they could modulate inflammation and immune function at a molecular level. To this end, the present review summarises preclinical and clinical experiences to provide the reader with an update on the effects of different macro- and micronutrients and dietary phenols in SLE, focusing on the mechanisms of action involved (Tables 1, 2 and 3).
Table 1 Beneficial effects of macronutrients in systemic lupus erythematosus (SLE)
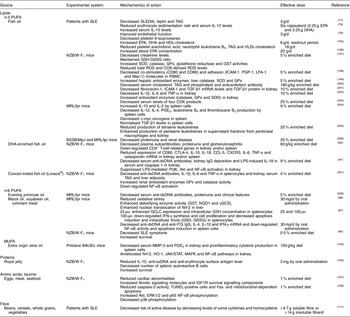
Akt, protein kinase B; CCL-5, chemokine (C-C motif) ligand 5; COX, cyclo-oxygenase; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; CXCR3, C-X-C motif chemokine receptor-3; dsDNA, double-stranded DNA; ERK, extracellular signal-regulated protein kinase; GCLC, γ-glutamylcysteine ligase catalytic subunit; γGCS, γ glutamyl cysteine synthetase; GPx, glutathione peroxidase; GSH, reduced glutathione; GSSG, oxidised glutathione; GST, glutathione-S-transferase; HO-1, haem oxygenase-1; ICAM-1, intercellular adhesion molecule-1; IGF1R, insulin-like growth factor receptor-1; IFN, interferon; JAK, Janus kinase; LFA-1, lymphocyte function-associated antigen-1; LPS, lipopolysaccharide; Mac-1, macrophage-1 antigen; MAPK, mitogen-activated protein kinase; MMP-3, metalloproteinase-3; MRL, Murphy Roths large; NQO1, NAD(P)H: quinone oxidoreductase-1; Nrf2, nuclear factor E2-related factor-2; NZB/W, New Zealand black/white; PBMC, peripheral blood mononuclear cell; PGP-1, permeability glycoprotein-1; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; SLEDAI, SLE disease activity index; SOD, superoxide dismutase; ssDNA, single-stranded DNA; STAT, signal transducer and activator of transcription; TGF, transforming growth factor; tTG, tissue transglutaminase; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labelling.
Table 2 Beneficial effects of micronutrients in systemic lupus erythematosus (SLE)
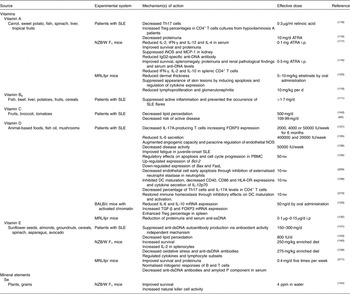
ATRA, all-trans-retinoic acid; Bax, BCL2 associated X protein; Bcl-2, B-cell lymphoma 2; DC, dendritic cell; dsDNA, double-stranded DNA; FasL, Fas ligand; FOXP3, forkhead box P3; HLA-DR, human leucocyte antigen – antigen D related; IFN, interferon; iNOS, inducible NO synthase; i.p., intraperitoneally; MCP-1, monocyte chemoattractant protein-1; MRL, Murphy Roths large; NOS, NO synthase; NZB/W, New Zealand black/white; PBMC, peripheral blood mononuclear cell; ppm, parts per million; ssDNA, single-stranded DNA; TGF, transforming growth factor; Th, T-helper; Treg, regulatory T cell.
Table 3 Beneficial effects of phenolic compounds in systemic lupus erythematosus (SLE)

2-OH, 2-hydroxyestrone; 16α-OH, 16α-hydroxyestrone; Akt, protein kinase B; AMPK; adenosine 5'-monophosphate-activated protein kinase; ANA, antinuclear antibodies; BAFF, B-cell activating factor; BW, body weight; COX, cyclo-oxygenase; DC, dendritic cell; dsDNA, double-stranded DNA; EGCG, epigallocatechin gallate; FOXP3, forkhead box P3; GPx, glutathione peroxidase; IFN, interferon; iNOS, inducible NO synthase; i.p., intraperitoneally; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; MRL, Murphy Roths large; mTOR, mammalian target of rapamycin; NLRP3, NLR family pyrin domain containing 3; Nrf-2, nuclear factor E2-related factor-2; NZB/W, New Zealand black/white; PI3K, phosphoinositide 3-kinase; ppm, parts per million; RNP, ribonucleoprotein; ROS, reactive oxygen species; SDG, secoisolariciresinol diglucoside; SNF, sucrose non-fermenting; ssDNA, single-stranded DNA; TGF, transforming growth factor; Th, T-helper; Treg, regulatory T cell.
Methods: literature search strategy
A literature survey was conducted to obtain published literature to create this review. The original search was conducted in April 2016 and a second, updated search was completed in October 2016. We searched MEDLINE and SCOPUS (January 1976, to present) databases with the terms ‘systemic lupus erythematosus’ and ‘lupus’ in combination with the terms ‘aetiology’, ‘apigenin’, ‘classification’, ‘carbinol’, ‘curcumin’, ‘diet’, ‘epidemiology’, ‘fibre’, ‘isoflavone’, ‘flavanols’, ‘flavones’, ‘flavonoids’, ‘flavonol’, ‘lignans’, ‘lipids’, ‘management’, ‘melatonin’, ‘mineral elements’, ‘natural products’, ‘nutrition’, ‘olive oil’, ‘pathogenesis’, ‘polyphenol’, ‘protein’, ‘resveratrol’, ‘stilbene’, ‘treatment’, ‘vitamins’, with no language restrictions. We also searched the references of articles identified by this strategy and selected those that were relevant.
Macronutrients in systemic lupus erythematosus
Lipids
Among traditional atherosclerotic risk factors in SLE, dyslipidaemia is believed to decisively affect the long-term prognosis of patients with SLE, not only with regard to cardiovascular events but also by influencing other manifestations, such as lupus nephritis and brain damage among others( Reference Tselios, Koumaras and Gladman 70 ). Therefore, dyslipidaemia in SLE should be prompt and adequately treated in order to reduce overall morbidity and mortality and improve patients’ quality of life. Moreover, dyslipaemia is considered as a modifiable risk factor that can be managed with an accurate treatment, which includes the promotion of a healthy and varied diet( Reference Tselios, Koumaras and Gladman 70 – Reference Borba and Bonfá 72 ).
Specifically, there is both preclinical and clinical evidence that patients with SLE may benefit from the consumption of certain lipids. In particular, the n-3 PUFA possess the most potent immunomodulatory activity; among them, EPA and DHA are the most biologically active. These n-3 PUFA suppress proinflammatory cytokine production, lymphocyte proliferation, cytotoxic T-cell activity, macrophage-mediated cytotoxicity and neutrophil/monocyte chemotaxis( Reference Calder 73 ). Some of the effects of n-3 PUFA are produced by the modulation of the amount and types of eicosanoids, but other effects have eicosanoid-independent mechanisms, including modulation of intracellular signalling pathways, transcription factor activity and gene expression( Reference Simopoulos 74 ). As humans and other mammals require PUFA but cannot synthesise them, the consumption of PUFA through the daily diet is essential. The available data show that an increased daily intake of dietary n-3 PUFA decreased the severity of autoimmune disorders. A low intake of n-3 PUFA and high intake of carbohydrate in SLE appear to be associated with worse disease activity, adverse serum lipids and plaque presence( Reference Elkan, Anania and Gustafsson 75 ). Similarly, consumption of fish oil, which is rich in n-3 PUFA, has been shown to be useful in preventing and/or ameliorating SLE symptomatology. In fact, different clinical studies have assessed the association between dietary supplements and fish oil in patients with SLE, revealing a number of positive effects, including reducing cardiovascular, inflammatory and neuromotor symptoms and improving depressive symptoms and fatigue( Reference Arriens, Hynan and Lerman 76 – Reference Duffy, Meenagh and McMillan 79 ). In addition, n-3 PUFA from fish oil have demonstrated anti-inflammatory effects when used as supplements in murine SLE models, decreasing autoantibody production and inflammatory gene expression in the kidney and spleen, suppressing glomerulonephritis and extending lifespan( Reference Pestka, Vines and Bates 80 , Reference Halade, Rahman and Bhattacharya 81 ). However, a major question in the use of PUFA is whether the doses required to suppress autoimmunity and inflammation could have a negative impact on innate and adaptive immune responses against pathogens or neoplastic events( Reference Fenton, Hord and Ghosh 82 ).
Conjugated linoleic acid (CLA) is the term used to describe positional and geometric isomers of the n-6 PUFA linoleic acid (18 : 2n-6). The main sources of CLA in the human diet are ruminant meat and dairy products, which are extensively used in Western diets, but it can be also found in small amounts in oils derived from plants( Reference Chin, Liu and Storkson 83 , Reference Hennessy, Ross and Fitzgerald 84 ). CLA has shown potential anti-inflammatory and cancer-preventive properties, most probably through its ability to modify eicosanoid signalling by altering cell membrane composition and modulate genes through PPAR( Reference Pariza, Park and Cook 85 , Reference Bassaganya-Riera, Hontecillas and Beitz 86 ). CLA has shown beneficial effects in SLE mouse models, reducing splenomegaly, autoantibody and cytokine production, NF-κB activity (independent of PPAR-γ activation) and oxidative stress, thus improving SLE symptoms and survival rates( Reference Bergamo, Luongo and Maurano 87 – Reference Yang and Cook 89 ). Moreover, some animal studies have shown that CLA consumption has anti-sclerotic and anti-oxidative effects and improves the blood lipid profile, which could be also beneficial for SLE( Reference Kritchevsky, Tepper and Wright 90 , Reference McLeod, LeBlanc and Langille 91 ). On the other hand, a previous study suggested that dietary CLA may accelerate the appearance of autoimmune symptoms in NZB/W F1 mice, despite protecting against disease-related body-weight loss and prolonged survival( Reference Yang, Pariza and Cook 92 ). By the way, only a few studies examined the effects of CLA in human subjects in vivo and their results do not show the favourable results observed in animal studies( Reference Lambert, Goedecke and Bluett 93 – Reference Nugent, Roche and Noone 95 ). In this sense, the majority of the clinical studies did not provide conclusive evidence for the effectiveness of CLA on human health, except for anti-obesity properties( Reference Gaullier, Halse and Høivik 96 , Reference Carvalho, Uehara and Rosa 97 ). Although the interest in CLA still remains, there are multiple questions related to the safety and efficacy on its consumption that must be scientifically solved( Reference Benjamin, Prakasan and Sreedharan 98 ).
Controversially, an essential fatty acid-deficient diet has shown beneficial effects in NZB/W F1 mice by reducing levels of arachidonic acid (20 : 4n-6), a precursor of proinflammatory eicosanoids, PG and leukotriene metabolites, decreasing autoantibody production and improving nephritis( Reference Harbige 99 ). Furthermore, an increased intake of n-6 PUFA may increase the production of proinflammatory cytokines and the incidence of autoimmune diseases by increasing free radicals and decreasing antioxidant enzyme mRNA levels( Reference Lin, Jeng and Chiang 100 ).
Recent experimental and clinical studies have confirmed that regular extra virgin olive oil (EVOO) consumption can exert positive effects in rheumatic diseases( Reference Rosillo, Alcaraz and Sánchez-Hidalgo 101 , Reference Rosillo, Sánchez-Hidalgo and Sánchez-Fidalgo 102 ). The beneficial properties of EVOO are linked to its high MUFA content and also to its multiple minor components, particularly polyphenol compounds such as flavonoids, lignans and secoiridoids and their hydrolysis products (hydroxytyrosol and tyrosol, among others). Recently, we evaluated the effects of an EVOO diet in a pristane-induced SLE model in mice, and we demonstrated that the EVOO diet significantly reduced renal damage and decreased metalloproteinase (MMP)-3 serum and PGE2 kidney levels as well as proinflammatory cytokine production in splenocytes. In addition, our data indicated that Nrf-2 and haem oxygenase protein expression was up-regulated by the EVOO diet and that the activation of the Janus kinase and signal transducer and activator of transcription (JAK/STAT), mitogen-activated protein kinases (MAPK) and NF-κB pathways was markedly ameliorated, supporting the interest in EVOO as a beneficial functional food exerting a preventive/palliative role in the management of SLE( Reference Aparicio-Soto, Sánchez-Hidalgo and Cárdeno 103 ).
Proteins
The restriction of dietary protein has been shown to have beneficial effects in controlling renal disease progression in animal models and human studies. Specifically, a protein-restricted diet (0·6 g/kg per d) improved nutritional status and glomerular filtration rate in patients with SLE with chronic kidney disease( Reference Milovanov, Lysenko and Milovanova 104 ). In addition, excessive protein intake has been shown to produce bone mineral loss in patients with juvenile SLE( Reference Caetano, Ortiz and Terreri 105 ). By contrast, oral supplementation with royal jelly, a honeybee secretion rich in proteins, free amino acids, SCFA and vitamins, has also been considered beneficial because its reduces cholesterol and has immunomodulating and anti-inflammatory activities. In fact, the intake of 2 mg of protein from royal jelly induced a reduction of IL-10, anti-single-stranded DNA and anti-erythrocyte surface antigen autoantibody serum levels, decreased the number of splenic autoreactive B cells and increased survival in NZB/W F1 mice, suggesting a beneficial effect in preventing the early onset of SLE and in controlling the active progression of SLE manifestations( Reference Mannoor, Shimabukuro and Tsukamotoa 106 ).
Taurine, the major intracellular free β-amino acid in most mammalian tissues, is obtained largely from the diet through eggs, meat and seafood, and it plays crucial roles in protecting biological systems owing to its antioxidant, anti-inflammatory and anti-apoptotic properties. The potential benefits of taurine intake in SLE have been demonstrated in an in vivo model of NZB/W F1 mice fed with a cholesterol-rich diet supplemented with taurine (1 %); taurine supplementation ameliorated cardiac abnormalities with reductions in aggravated histopathological changes, Fas- and mitochondrial-dependent apoptosis and fibrotic signalling molecules and an increase in insulin-like growth factor receptor 1 survival signalling components( Reference Huang, Hsu and Kuo 107 ). In another study with NZB/W F1 mice that were fed a cholesterol-rich diet supplemented with taurine (1 %), taurine significantly reduced caspase-3 activity, terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)-positive cells and Fas- and mitochondrial-dependent apoptosis, increased phosphorylation of protein kinase B (Akt), extracellular signal-regulated protein kinases 1 and 2 and p65 NF-κB proteins and decreased phosphorylation of p38 protein in the liver. These results strongly suggest the therapeutic potential of taurine intake in SLE management( Reference Hsu, Chiang and Wu 108 ). By contrast, an increased intake of the amino acid l-arginine increased the severity of renal fibrosis and the likelihood of death in Murphy Roths large (MRL)/lpr mice by enhancing cytotoxic NO generation via inducible NO synthase (iNOS)( Reference Peters, Border and Rückert 109 ). Moreover, a diet with restricted phenylalanine and tyrosine had beneficial effects on NZB/W F1 mice by decreasing autoantibody production( Reference Corman 110 ).
Fibre
An adequate intake of dietary fibre is recommended in SLE because of the beneficial effects of fibre in decreasing cardiovascular risk, promoting gut mobility and reducing serum levels of inflammation markers such as C-reactive protein, cytokines and homocysteine. A study with 279 women with SLE showed an inverse association between dietary fibre intake and SLE; increased dietary fibre decreased the risk of active disease in patients with SLE by decreasing the levels of some cytokines and homocysteine( Reference Minami, Hirabayashi and Nagata 111 ). However, excessive fibre intake may reduce the absorption of vitamins, minerals and proteins( Reference Klack, Bonfa and Borba Neto 59 ).
Micronutrients
Vitamins
Several epidemiological studies have explored the potential role of antioxidant nutrient intake and supplementation in patients with SLE. In SLE, oxidative stress acts as an autoimmunity trigger that contributes to immune system dysregulation, abnormal apoptotic events and autoantibody production. Levels of oxidative stress have been shown to be correlated with SLE disease activity and organ damage in patients and in SLE-prone mouse models( Reference Gergely, Niland and Gonchoroff 112 , Reference Bethunaickan, Sahu and Liu 113 ). In addition to spreading inflammation through the bloodstream, mediators of oxidative stress contribute to organ damage, including the cardiovascular system, kidneys and skin. Thus, suppressing pathways of oxidative stress may reduce the toxicity of immunosuppressive therapies, decrease disease activity and improve the quality of life of patients with SLE( Reference Perl 114 ).
Vitamin A is essential for multiple functions, including the maintenance of immune system integrity, and its physiological form, all-trans-retinoic acid (ATRA), regulates gene transcription by binding to nuclear retinoic acid receptors. ATRA has demonstrated paradoxical effects on the development of autoimmune lupus in the MRL/lpr mouse, decreasing inflammation in some organs while generating more severe disease in others( Reference Liao, Ren and Wei 115 ). However, ATRA has also shown beneficial effects, alone or in combination with low-dose immunosuppressive drugs, in lupus nephritis and cytokine modulation in both mouse models and patients with SLE, suggesting that ATRA treatment significantly alleviates hyper-reactive autoimmune and renal disorder, most likely via cytokine regulation( Reference Kinoshita, Kishimoto and Shimazu 116 – Reference Pérez de Lema, Lucio-Cazaña and Molina 119 ). Furthermore, oral treatment with etretinate, a synthetic vitamin A derivative, every 2 d for 2 months in lupus mice reduced dermal thickness through the inhibitory effects on collagen synthesis and inhibited skin lesions by inducing apoptosis in dermal infiltrating cells and, perhaps, regulating cytokine production( Reference Ikeda, Nishide and Ohtani 120 ).
Higher serum levels of homocysteine are associated with atherosclerotic vascular events in patients with SLE. In this sense, an increase of one log unit of homocysteine concentrations led to a 2·4-fold increase in the risk of stroke, and a 3·5-fold increase in the risk of arterial thrombosis in SLE( Reference Petri, Roubenoff and Dallal 121 ). In this regard, dietary intake of vitamin B6, which has been shown to decrease homocysteine levels and acts as a coenzyme in the metabolism of antibodies and cytokines, decreased the risk of active SLE in female patients, suggesting that its daily intake may lead to the suppression of active inflammation and prevent the occurrence of SLE flares. However, in the present study there was no significant association between B6 intake and a decreased risk of atherosclerotic vascular events( Reference Minami, Hirabayashi and Nagata 111 ).
Vitamin C may mediate the oxidative stress response in SLE and, consequently, exert beneficial effects on the repair of abnormal immune components and inflammation. Vitamin C intake (500 mg/d for 6 weeks) may positively modulate oxidative DNA damage in healthy subjects, but these positive effects were not observed in patients with SLE. These significant differences in the response to vitamin C supplementation between patients with SLE and healthy subjects might be associated with DNA repair abnormalities observed in SLE( Reference Evans, Cooke and Akil 122 ). Another study reported that vitamin C intake (109·99 mg/d) is inversely associated with the risk of active SLE, decreasing oxidative stress and suppressing autoantibody production, suggesting that its intake may prevent the occurrence of SLE flares( Reference Minami, Sasaki and Arai 60 ).
Vitamin D is found in small quantities in eggs, fatty fish, and supplemented dairy products; however, the main source of vitamin D is sunlight exposure( Reference Dusso and Brown 123 ).Vitamin D has a critical role in Ca homeostasis, mineralisation of bone tissue, muscle function and coordination. Indeed, low levels of vitamin D result in a decrease of the Ca reserves in bone in an attempt to correct for the reduced Ca that will be absorbed from the gut( Reference Lane 124 ). Nowadays it is clear that vitamin D deficiency contributes to the morbidity and mortality of multiple chronic diseases, including SLE( Reference Kamen and Aranow 125 ). Particularly, vitamin D deficiency is even more prevalent in patients with SLE than in the general population, in part because patients with SLE are recommended to avoid the sun, in order to prevent disease flares( Reference Borba, Vieira and Kasamatsu 126 ). In this sense, some studies have shown that vitamin D deficiency increases predisposition to suffer SLE partly owing to the photosensitive nature of the disease, exacerbates symptoms, decreased bone health and it is correlated with the disease activity( Reference Marques, Dantas and Fragoso 127 – Reference Casella, Seguro and Takayama 129 ). Moreover, in a study with adolescent women with SLE supplemented with Ca and vitamin D (400–800 IU daily), a lack of vitamin D was associated with a decrease of bone mineral density, highlighting the importance of well-defined vitamin D supplementation protocols in SLE( Reference Caetano, Terreri and Ortiz 130 ). Vitamin D supplementation (2000, 4000 or 50000 IU weekly for 6 months) in patients with SLE showed positive immunological effects by decreasing IL-17A-producing T cells and increasing FOXP3 expression, confirming the relationship between vitamin D status and immunological balance( Reference Marinho, Carvalho and Boleixa 131 ). Furthermore, vitamin D consumption attenuated the disease progression and increased the number of regulatory T cells in mouse models( Reference Lemire, Ince and Takashima 132 , Reference Lavi Arab, Rastin and Faraji 133 ). In patients with SLE, in vitro treatment with vitamin D (50 nm) has regulatory effects on apoptosis and cell cycle progression and modifies the expression levels of apoptotic genes( Reference Tabasi, Rastin and Mahmoudi 134 ); in addition, vitamin D intake (400 000 IU followed by 20 000 IU weekly for 12 weeks) can positively modulate endothelial function in patients with stable SLE, reducing cardiovascular risk( Reference Reynolds, Haque and Williamson 135 ). Moreover, vitamin D supplementation (50 000 IU per week for 24 weeks) has been shown to be effective in decreasing disease activity and improving fatigue in juvenile-onset SLE( Reference Lima, Paupitz and Aikawa 136 ). Therefore, vitamin D supplementation is currently recommended as a treatment for some patients with SLE, although determining the effectiveness and safety of vitamin D use as a treatment in SLE, as well as establishing the exact doses, still requires additional extensive interventional studies( Reference Azrielant and Shoenfeld 137 ). Nevertheless, general international recommendations have established that vitamin D supplementation with 800 to 1000 IU/d or 50000 IU monthly is safe for most individuals and can ensure levels of vitamin D within the optimal range. This intake is within the currently recommended safe upper tolerable limit for vitamin D of 2000 IU/d for those aged 1 year and older( Reference Kennel, Drake and Hurley 138 ).
Vitamin E is a potent free radical and peroxyl radical scavenger and an essential nutrient for maintaining the normal function of the immune system. A diet supplemented with vitamin E decreased oxidative stress and anti-dsDNA antibody production, regulated cytokines and lymphocyte subsets and alleviated the severity of SLE under oxidative stress in NZB/W F1 mice( Reference Hsieh and Lin 139 ). However, while low vitamin E supplementation increased the survival of MRL/lpr mice, high supplementation had the opposite effect and inhibited the Th1 pathway, which may not be beneficial for Th2-prone autoimmune diseases, such as SLE. This duality might be due to selective modulation through PGE2 production or through PPARγ, inhibitor of NF-κB (IκB-α) and apoptotic pathways under different doses of vitamin E( Reference Hsieh and Lin 139 , Reference Hsieh and Lin 140 ). In patients with SLE, the oral administration of vitamin E (150–300 mg/d) together with prednisolone decreased autoantibody production but not the urinary levels of 8-hydroxydeoxyguanosine, an indicator of oxidative DNA damage, suggesting that vitamin E can suppress autoantibody production via a mechanism independent of antioxidant activity( Reference Maeshima, Liang and Goda 141 ).
Despite the aforementioned evidence, a survey study showed that the dietary intake of antioxidant nutrients, including vitamins A, C and E, α-carotene, β-carotene, cryptoxanthin, lycopene and lutein, from foods and supplements does not change the risk of developing SLE in women, suggesting that antioxidants do not offer protection against SLE( Reference Costenbader, Kang and Karlson 142 ). In addition, a study investigating the effects of vitamin E and C supplementation (500 mg vitamin C and 800 IU vitamin E daily) on endothelial function and markers of oxidative stress and antioxidant defence in patients with SLE showed that their combined administration decreased lipid peroxidation but did not affect endothelial function after 3 months of therapy( Reference Tam, Li and Leung 143 ). Insufficient dosing and/or the inability of these vitamins to regulate intracellular signalling pathways within the immune system may explain this lack of therapeutic efficacy.
Mineral elements
Se, a trace mineral widely distributed in plants and grains, is a required nutrient for animals and humans that has been shown to have immunological and anti-inflammatory properties. Se supplementation (sodium selenite 4 parts per million (ppm) in the drinking water) demonstrated beneficial effects in female NZB/W F1 mice, improving survival and increasing natural killer (NK) cell activity. However, Se supplementation had no effect on autoantibody production( Reference O’Dell, McGivern and Kay 144 ).
Nowadays, it is recommended that patients with SLE follow a Na-restricted diet because evidence suggests that excess sodium chloride content in the diet might be a potential risk factor for autoimmune diseases. A study in MRL/lpr mice demonstrated that excessive dietary sodium chloride intake aggravated lupus nephritis through the effect of the inducible serine/threonine protein kinase 1 pathway on the Th1/Th2 and Th17/Treg balance in vivo ( Reference Yang, Yao and Chen 145 ).
Dietary restriction of Zn also has shown beneficial effects in SLE. Moderate (5·0 ppm daily) to severe (2·5 ppm daily) dietary restriction of Zn and decreased energy intake in NZB/W F1 mice decreased the onset of haemolytic anaemia, autoantibody production and renal disease( Reference Corman 110 ). In MRL/lpr mice, a Zn-restricted diet has been shown to reduce lymphoproliferation and anti-dsDNA titres and improve glomerulonephritis( Reference Brown 146 ).
Dietary phenols in systemic lupus erythematosus
Polyphenols
Polyphenols are common constituents of plant-based foods and the main dietary source of antioxidants through vegetables, fruits, cereals, legumes and drinks such as tea, coffee and wine. The term polyphenol comprises a wide variety of molecules with phenolic structural features that are classified into several groups according to their number of phenol rings and the structural elements that bind these rings together. The main categories of polyphenols are phenolic acids, flavonoids, diarylheptanoids and arylalkanones lignans and stilbenes. There is marked variation in bioavailability among polyphenols, mainly owing to differences in chemical structure. Furthermore, the most abundant polyphenols in our diet are not necessarily those that present the best bioavailability profile( Reference D’Archivio, Filesi and Di Benedetto 147 ).
In recent years, the interest in dietary polyphenols has increased considerably among nutritionists and food scientists owing to their beneficial roles in human health; polyphenols act as anti-inflammatory, anti-cancerous and immunomodulatory agents in several diseases. The potential beneficial effects of polyphenols in SLE are derived from their abilities to protect against oxidative damage, modulate different inflammation-related enzymes and interact with signal-transduction pathways, cell cycle regulators and cell receptors implicated in the immunoinflammatory process( Reference Cárdeno, Sánchez-Hidalgo and Alarcón-de-la-Lastra 65 , Reference D’Archivio, Santangelo and Scazzocchio 148 ). Evidence from both in vitro and in vivo studies demonstrates the beneficial effect of phenolic compounds in the management of SLE (Table 3).
Flavonoids: flavones
Apigenin, a flavonoid widely distributed in dietary plants such as parsley, thyme and chamomile, among others, has vasorelaxing, antiplatelet and antioxidant properties, which may reduce the risk of coronary disease and improve endothelial function in SLE( Reference Woodman and Chan 149 , Reference Kang, Ecklund and Liu 150 ). Apigenin has shown beneficial effects in an SLE mouse model, suppressing autoantibody production and the IFN-γ and IL-17 response of stimulated splenocytes, IL-6 production by lupus dendritic cells and inducible isoform of cyclo-oxygenase-2 (COX-2) expression in CD4+ T cells, B cells, dendritic cells and macrophages. Both IFN-γ-producing Th1 cells and IL-17-producing Th17 cells are critical for help in the production of pathogenic autoantibodies and the development of lupus nephritis( Reference Hsu, Yang and Wang 151 , Reference Haas, Ryffel and Le Hir 152 ). In addition, apigenin treatment in vitro induced significant apoptosis of T cells, B cells, dendritic cells and macrophages after 24 h of incubation( Reference Kang, Ecklund and Liu 150 ). Nevertheless, the presence of apigenin in the diet is insufficient to reach bioavailable therapeutic levels owing to first-pass metabolism in the gut and liver, but its bioavailability could potentially be improved by the pharmaceutical industry.
Flavonoids: flavonols
Astilbin, a natural flavonol found in some food and medicinal plants, exhibits multiple pharmacological functions, including anti-inflammatory and immunomodulatory properties through the induction of apoptosis in activated T cells and the suppression of activated T-cell adhesion and migration and modulation of dendritic cells. Oral administration of astilbin, isolated from the rhizome of Smilax glabra Roxb (family Liliaceae), delayed disease development in lupus-prone mice when preventive oral administration was started before the onset of disease and also when the treatment was started after disease onset. In addition, astilbin treatment reduced levels of circulating antinuclear antibodies and several serum cytokines and decreased functional activated T and B cells, suggesting that astilbin treatment decreases the capacity of B cells to stimulate T lymphocytes( Reference Guo, Liu and Lu 153 ).
Flavonoids: flavanols
Several studies suggest that the regular consumption of tea, the most widely consumed beverage in the world after water, is associated with an array of health benefits. In particular, epidemiological studies indicate that the incidence of death due to heart, cerebrovascular and respiratory diseases is considerably lower in China and Japan, the two leading green tea-consuming countries( Reference Khan and Mukhtar 154 – Reference Hsu and Dickinson 156 ). All tea is derived from Camellia sinensis (L.) kuntze, an evergreen shrub of the Theaceae family, whose health benefits are mainly associated with its high concentration of flavanols known as catechins( Reference Khan and Mukhtar 154 ). Epigallocatechin gallate (EGCG), the major bioactive polyphenol present in green tea, has been reported to have antioxidant and anti-inflammatory effects by inhibiting NF-κB and suppressing T-cell activation( Reference Singh, Shankar and Srivastava 157 ). A number of studies have proved the beneficial effects of green tea or EGCG supplementation in SLE models. An in vivo study demonstrated that a diet supplemented with green tea powder increased mouse survival and improved SLE progression by decreasing serum levels of anti-DNA antibodies and renal damage( Reference Sayama, Oguni and Tsubura 158 ). Furthermore, daily treatment with EGCG by oral administration in lupus-prone mice had prophylactic effects by promoting the Nrf-2 antioxidant signalling pathway, inhibiting NLR family pyrin domain-containing 3 (NLRP3) inflammasome activation in the kidney and enhancing Treg activity( Reference Tsai, Ka and Chang 159 ). The potential therapeutic role of EGCG has also been studied in mesangial cells, which are macrophage-like cells resident in the kidney with immune and vascular functions, from lupus mice pretreated with EGCG and stimulated with lipopolysaccharide (LPS) or IFN-γ. EGCG activated the metabolic regulator adenosine 5'-monophosphate-activated protein kinase (AMPK), which has been shown to inhibit the production of several proinflammatory mediators, and blocked the induced expression of iNOS and NO and IL-6 production via a mechanism partially independent of the AMPK activation. Furthermore, EGCG attenuated inflammation via the immune-stimulated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K-Akt-mTOR) pathway by decreasing phosphorylation of the kinase Akt( Reference Peairs, Dai and Gan 160 ). EGCG has also been reported to decrease the expression of different autoantigens at the mRNA and/or protein levels in an in vivo model of normal human primary epidermal keratinocytes, a cell type particularly affected in patients with SLE, reducing the expression of several antinuclear autoantibodies( Reference Hsu, Dickinson and Qin 161 ). All these data suggest the potential therapeutic role of EGCG as an important component in novel approaches to SLE management by regulating inflammation and preventing lupus nephritis.
Flavonoids: isoflavones
Nowadays, it is clear that high oestrogen levels exacerbate SLE symptoms and kidney disease and increase numbers of autoreactive B cells and autoantibodies( Reference Fairweather, Frisancho-Kiss and Rose 162 ). Phyto-oestrogens are plant-derived compounds that are found in a wide variety of foods and that are structurally and/or functionally similar to mammalian oestrogens and their active metabolites. Phyto-oestrogens can interact with oestrogen receptors (ER) with oestrogenic or anti-oestrogen activities. Genistein and daidzein are the major bioactive isoflavones, and they are mainly available in soya, the cornerstone of the traditional Asian diet. Soya has shown anti-inflammatory effects and is able to reduce proteinuria and renal pathological lesions associated with progressive renal failure, which could be beneficial for SLE. In vivo studies showed that dietary supplementation with soya isoflavones extracted from natural soya germ, containing 50 % daidzin, 30 % glycitin and 20 % genistein, alleviated disease severity in lupus mice and decreased autoantibody levels and in vitro IFN-γ production in splenocytes. Furthermore, methanol extracts of soya isoflavones demonstrated a higher affinity for ERβ than ERα, suggesting that ER activation is selectively modulated( Reference Hong, Wang and Huang 163 ). These results are consistent with those of previous studies, which proved that SLE disease severity is aggravated by ERα agonists but ameliorated by ERβ agonists in a murine lupus model( Reference Li and McMurray 164 ). By contrast, a previous study showed that a diet supplemented with 20 % soyabean protein and 5 % soyabean oil exacerbated the SLE clinical course in autoimmune MRL/lpr mice, decreasing survival and increasing renal damage, thymus weight and T-cell proliferation in the spleen and B cells in lymph nodes. Thus, further research on the mechanism underlying the effect of soya-rich diets is necessary to evaluate the potential for isoflavone supplementation in patients with SLE( Reference Zhao, Sun and Horiguchi 165 ). However, in recent years isoflavone research in SLE has not evolved so far. Even though animal data showed that isoflavones have a wide range of molecular, cellular and behavioural effects at doses and plasma concentrations attainable in humans, the consumption of soya or soya phyto-oestrogen has produced mixed and often conflicting results and potential adverse effects on humans, which may limit their use in further studies( Reference Patisaul and Jefferson 166 , Reference Bedell, Nachtigall and Naftolin 167 ).
Coumestrol is the major phyto-oestrogen in lucerne sprouts but can be also found in beans and other vegetables. Dietary supplementation with coumestrol in SLE mice ameliorated some aspects of the disease( Reference Schoenroth, Hart and Pollard 168 ). Furthermore, a diet supplemented with lucerne sprout ethyl acetate extract significantly improved proteinuria, prolonged lifespan and suppressed the production of proinflammatory cytokines in stimulated peritoneal cells and splenocytes from SLE mice. However, there was no change in SLE autoantibody production, suggesting that its beneficial effects might be more attributable to the down-regulation of inflammatory cytokines rather than autoantibody regulation. Nevertheless, a group of mice fed a diet supplemented with lucerne sprouts had similar proteinuria progression and lifespan as the lupus group, suggesting that the ingestion of whole lucerne sprouts had no beneficial effects on SLE. Accordingly, lucerne sprout ethyl acetate extract reduced serum levels of proinflammatory cytokines and improved survival after 9 h of LPS challenge, suggesting that lucerne sprout ethyl acetate extract alleviated acute inflammatory hazards. Moreover, pretreatment with lucerne sprout ethyl acetate extract significantly inhibited proinflammatory cytokine production in LPS-stimulated primary macrophage( Reference Hong, Chao and Chen 169 , Reference Hong, Huang and Wang 170 ). By contrast, previous reports have shown that lucerne seed or sprouts can induce SLE-like disease in monkeys and exacerbate disease severity in patients with SLE who have ingested lucerne tablets( Reference Malinow, Bardana and Pirofsky 171 , Reference Roberts and Hayashi 172 ).
Lignans
Flaxseed (Linum usitatissimum L.), the richest source of lignan precursors, has been a part of the human diet for thousands of years. The principal dietary lignan precursor in flaxseed is the secoisolariciresinol diglucoside (SDG), which has demonstrated possible nutraceutical actions to prevent and alleviate lifestyle-related diseases( Reference Imran, Ahmad and Anjum 173 ). A flaxseed-supplemented diet in a lupus mouse model improved renal damage and decreased splenic lymphocyte proliferation( Reference Hall, Parbtani and Clark 174 ). Similarly, oral administration with SDG from flaxseed showed dose-dependent protective effects in the kidney, with similar effects to those previously noted for dietary supplementation with flaxseed( Reference Clark, Muir and Westcott 175 ). In a randomised cross-over trial, flaxseed supplementation over 17 weeks was well tolerated by patients with lupus nephritis and exerted significant positive effects on renal function( Reference Clark, Parbtani and Huff 176 ). In another randomised cross-over trial, daily supplementation with flaxseed in patients with SLE appeared to be renoprotective at the end of the 2-year study( Reference Clark, Kortas and Heidenheim 177 ).
Diarylheptanoids and arylalkanones
Curcumin (1,7-bis-[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione), a component of the rhizomes of the golden spice turmeric (Curcuma longa L.), has demonstrated antioxidant, anti-tumour and anti-inflammatory activities. Recent studies have suggested that curcumin may have therapeutic effects in SLE treatment. In in vitro studies, low doses of curcumin modulated the Th17/Treg balance of CD4+ T cells isolated from patients with SLE without affecting CD4+ T cells of healthy subjects. As SLE pathogenesis involves the enhancement of Th17 cells with a parallel reduction in the Treg population in patients with SLE, the regulation of this balance has become a subject of interest in SLE management, which is intended to improve the clinical outcome and prevent organ damage( Reference Handono, Pratama and Endharti 178 ). Curcumin has also shown beneficial effects in SLE mouse models, producing a delayed onset of autoantibody production and proteinuria and significantly decreasing salivary gland infiltration and lymphadenopathy compared with the control group( Reference Kurien, Harris and Quadri 179 ). Moreover, a curcumin-supplemented diet improved renal damage and autoantibody and proinflammatory cytokine production in female lupus mice. Interestingly, these therapeutic effects disappeared after Treg depletion by anti-CD25 antibody injection, suggesting that the protective effects of curcumin involve, at least partially, its interaction with Tregs( Reference Lee, Kim and Lee 180 ). In addition, a randomised and placebo-controlled study demonstrated that oral supplementation with curcumin (three capsules per d over 3 months, each capsule containing 500 mg of turmeric (22·1 mg of curcumin)) has beneficial effects without side effects in lupus patients with refractory nephritis, suggesting that short-term curcumin supplementation may be used safely as an adjuvant therapy in patients with SLE( Reference Khajehdehi, Zanjaninejad and Aflaki 181 ). However, curcumin (30 mg/kg body weight administered from 12 to 20 weeks by daily intraperitoneal injection) showed negative effects in the central nervous system of MRL/lpr mice, increasing IgG and anxiety behaviour and decreasing central complement protein (C3) deposits in the brain and circulation. Curcumin also worsened brain atrophy, reduced the volume of the hippocampus and increased the mRNA and protein expression of aquaporin 4, the main water channel of the brain, aggravating brain oedema. Furthermore, increased splenocyte proliferation was suggestive of peripheral immune response activation. Therefore, until a safe dose range is established by additional studies, caution is warranted in the use of curcumin even as adjuvant therapy for central nervous system lupus( Reference Foxley, Zamora and Hack 182 ).
Stilbenes
Resveratrol (3,5,4’-trihydroxystilbene) is a natural compound found in various plants and fruits, especially in grapes, that possess anti-inflammatory, immune-regulatory, antioxidant and blood fat-regulatory activities. In particular, resveratrol can modulate inflammatory genes and signalling transcription factors, such as STAT3, NF-κB and COX-2, with critical roles in SLE pathogenesis( Reference Nakata, Takahashi and Inoue 183 ). A recent study showed that resveratrol had protective effects in a pristane-induced lupus BALB/c mouse model after 7 months of treatment and in vitro immunomodulatory effects on splenic mononuclear cells, suggesting that it may represent a novel approach for the management of SLE( Reference Wang, Luo and Li 184 ).
Indole-3-carbinol
Indole-3-carbinol (I3C), the breakdown product of glucobrassicin, is obtained from the dietary consumption of cruciferous vegetables (family Brassicaceae), such as broccoli, cabbage, Brussels sprouts and cauliflower. An in vivo study showed that I3C dietary supplementation (0·2 g/kg) increased the lifespan of lupus-prone NZB/W F1 mice and decreased proteinuria, glomerulonephritis and interstitial nephritis. Furthermore, I3C supplementation was effective when initiated before or after disease onset and may be beneficial in SLE prevention as well as in the early stages of SLE. In addition, the ratio of the urine oestrogen metabolites 2-hydroxyestrone (2-OH) and 16α-hydroxyestrone (16α-OH) was greater after the I3C diet, showing anti-oestrogenic activities( Reference Auborn, Qi and Yan 185 ). Similarly, another study demonstrated that a diet enriched with 2000 ppm I3C preserved renal function by decreasing proteinuria and renal pathological abnormalities and prolonged survival when fed to NZB/W F1 mice for 40 weeks. I3C supplementation decreased anti-dsDNA, anti-chromatin and anti-RNA helicase A antibody levels; increased the ratio of 2-OH to 16α-OH metabolites by maintaining 2-OH oestrone levels; and blocked B- and T-cell maturation, inhibiting disease progression. In addition, in vitro concanavalin A stimulation of T cells from the spleen of I3C-diet mice produced Th1 cytokines (IL-2 and IFN-γ) in contrast to the higher production of Th2 cytokines (IL-4 and IL-10) in the SLE control animals( Reference Yan, Qi and Telusma 186 ). Oestrogen metabolism in women with SLE is weighted towards 16α-OH, which might increase SLE disease activity. In an open-label 1-week metabolic study in women with SLE, the consumption of 375 mg/d of I3C changed the ratio of urinary 16α-OH to 2-OH predominantly owing to an increase in 2-hydroxylation, demonstrating that I3C could play a role in the therapy of SLE by attenuating oestrogen-dependent disease activity( Reference McAlindon, Gulin and Chen 187 ). These studies provide a preliminary understanding of mechanisms whereby I3C leads to the amelioration of lupus-like disease and suggest that I3C may be an effective adjunctive therapy for the human disease.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine synthesised from the essential dietary amino acid tryptophan, which can be found in a several components of the diet, for example, fruits, vegetables, olive oil and nuts. Consuming such foods gives rise to a significant increase in serum melatonin levels( Reference Peuhkuri, Sihvola and Korpela 188 , Reference Sae-Teaw, Johns and Johns 189 ). Melatonin concentrations are decreased in lupus patients and inversely correlated with disease activity. Melatonin administration has shown immunomodulatory effects, stimulating the immune system under basal immunosuppressive conditions and inhibiting exacerbated immune responses( Reference Robeva, Tanev and Kirilov 190 ). Administration of melatonin to MRL/lpr mice at a dose of 30 mg/kg body weight in drinking water over 1 month improved the histological kidney pattern in females and worsened it in males. Moreover, female mice treated with melatonin showed a decrease in serum IgG, IgM, anti-dsDNA and anti-CII autoantibodies, proinflammatory cytokines (IL-2, IL-6, IFN-γ, TNF-α and IL-1β) and nitrite/nitrate production and an increase in anti-inflammatory cytokines (IL-10). However, in male mice the treatment with melatonin elicited the opposite effect, worsening all the immunological parameters with elevated autoantibodies titres and a prevalence of proinflammatory v. anti-inflammatory cytokines. Similar results were obtained when lymphocytes from the spleen and lymph nodes were cultured: the indoleamine decreased the proinflammatory cytokines and increased the anti-inflammatory cytokines produced by lymphocytes in females but had the opposite effect in males, suggesting that melatonin action in MRL/lpr mice is sex dependent, probably through the modulation and inhibition of sex hormones( Reference Jimenez-Caliani, Jimenez-Jorge and Molinero 191 ). In MRL/lpr mice treated with the same amount of melatonin and hormone therapy, adding testosterone to female mice and oestradiol to males, melatonin alone prevented lupus development in females, but the addition of testosterone to melatonin-treated female mice produced a similar negative pattern to that observed previously in male lupus mice. These effects were similar in oestradiol-treated males, confirming that melatonin action in MRL/lpr mice could be sex dependent through the modulation of sex hormones( Reference Jimenez-Caliani, Jimenez-Jorge and Molinero 192 ). Furthermore, melatonin treatment (0·01, 0·1, 1·0 mg/kg) in pristane-induced SLE BALB/c mice antagonised the increasing levels of IgM, anti-single-stranded DNA and histone autoantibodies and decreased renal lesions caused by pristane. In an in vitro experiment with concanavalin A- or LPS-stimulated splenocytes isolated from melatonin-treated mice, melatonin decreased induced IL-6 and IL-13 production and increased IL-2 levels, showing that the indoleamine could modulate the disturbance of the cytokine network in SLE mice by regulating the Th1/Th2 cytokine imbalance( Reference Zhou, Wei and Si 193 ). In vitro administration of melatonin (1×10–4 m) in peripheral mononuclear cells from treated patients with SLE increased the number of Tregs expressing FOXP3 and BAFF mRNA expression in patients with SLE, whereas it caused up-regulation of BAFF mRNA levels in healthy subjects, supporting the dual role of melatonin in the cells of patients v. controls and its potential use as an immunomodulatory therapy or co-therapy for SLE( Reference Medrano-Campillo, Sarmiento-Soto and Álvarez-Sánchez 194 ).
Conclusion
The increasing body of evidence linking the intake of different nutrients to the potential beneficial effects derived from their antioxidant, anti-inflammatory and immunomodulatory properties suggests a possible supportive role of diet therapy in the primary and secondary prevention and management of SLE. Over previous years, human and mouse models have supplied several dietary candidates and have shed light on the possible mechanisms underlying the response to different nutrients, with promising results.
Although the studies are not conclusive and further research is required, it seems to be clear that a balanced diet can be helpful in the prevention and management of SLE, contributing to the management of the disease activity as well as the reduction of co-morbidities, thus improving health and quality of life of patients with SLE. In particular, widespread evidence highlighted the importance of a diet rich in vitamins (mainly A, B6, C, D and E) and MUFA/PUFA (particularly n-3 PUFA and MUFA) with an adequate fibre intake, protein and Na restriction and moderate energy consumption in reducing co-morbidities and preventing SLE flares, thus minimising unnecessary burden in patients with SLE. It is also remarkable the promising role of dietary polyphenols included in the diet through vegetables, fruits, cereals, legumes and drinks such as tea and wine in the management of SLE. Likewise, it is important to encourage patients to stop smoking, avoid being overweight and optimise their blood pressure, lipid profile, and control of disease activity to decrease cardiovascular morbidity.
Currently, researchers continue to search and identify potential dietary candidates that could play beneficial roles in SLE. Nevertheless, despite the positive preliminary results obtained over the last years, most of the reported beneficial effects need further verification before they can be translated into clinical practice. The efficacy of these dietary candidates and other nutrients, as well as the effect of dietary interventions aimed at promoting adequate nutritional status in SLE patients, requires further evaluation through prospective and randomised trials in human subjects.
Acknowledgements
The present review was supported by research grants AGL 2011-26949 (Ministerio de Economía y Competitividad, ISCIII, Eur J Nutr 1 3 FEDER) and P-10AGR-6609 (Junta de Andalucía). M. A.-S. gratefully acknowledges support from a Postgraduate National Programme of FPU fellowship (FPU2012/03430) and financial sponsorship from the Spanish Ministerio de Educación, Cultura y Deporte.
M. A.-S., M. S.-H and C. A. designed and prepared the manuscript. All authors read and approved the final content of the manuscript.
The authors declare that they have no conflicts of interest.






