Introduction
The German coast is rich in Geest, a landform of sands, gravels and tills from the Pleistocene. With the melting of the continental ice sheets since the end of the Last Glacial Maximum about 20,000 years ago, the global sea level rose about 130 m until today (Lambeck et al., Reference Lambeck, Esat and Potter2002). After the rising sea had filled the North Sea basin and advanced up the slopes of the Geest, various kinds of coastal fens developed due to the rising groundwater level and deposited peat there. When the sea reached the fens, muddy lagoonal and later sandy marine sediments were deposited on top of the peat (Streif, Reference Streif2004). This was not a continuous process, but interrupted by phases of reduced marine influence, allowing the accumulation of fen peats once more on previously deposited lagoonal sediments (Behre, Reference Behre2007). Such recurring phases of peat formation left intercalated layers of different ages (Streif, Reference Streif2004). Hence, the lower slopes of the Geest in the Wadden Sea and the currently embanked hinterland are covered by several metres of Holocene peat, clay and sand layers, surmounted over by the Geest heights. The exact timing, rates and progress of sea-level rise are still under debate (Bungenstock et al., Reference Bungenstock, Freund and Bartholomä2021) and of crucial importance for any projections of landscape change under a predicted sea-level rise as a result of global warming (Golledge et al., Reference Golledge, Keller, Gomez, Naughten, Bernales, Trusel and Edwards2019). The WASA (The Wadden Sea as an archive of landscape evolution, climate change and settlement history: exploration – analysis – predictive modelling) interdisciplinary joint project emphasises the reconstruction of the late Quaternary development of the German Wadden Sea (see also Bittmann et al., Reference Bittmann, Bungenstock and Wehrmann2021). Here we focus on the wide ecological consequences and profound habitat changes driven by sea-level change during the past few millennia, offering a variety of natural resources for early human inhabitants for hunting, fishing and gathering in the Wadden Sea area (Bazelmans et al., Reference Bazelmans, Meier, Nieuwhof, Spek and Vos2012; Becker and Siegmüller, Reference Becker and Siegmüller2021).
Study area, materials and methods
The study area covers most of the back-barrier area and a few marine sites northwest of the East Frisian island of Norderney and within the UNESCO World Heritage-listed Wadden Sea area. The intertidal flats of the back-barrier area drain twice a day with the ebbing of the tide, while deeper channels and the sites further from the island are permanently flooded. The tidal range is about 2.4 m (Behre, Reference Behre2003) but may have been less in the past (Franken, Reference Franken1987). Cores were drilled on the tidal flats at low water using a vibrocorer system with 6 m long aluminium tubes with an inner diameter of 8 cm. All other cores were obtained from a research vessel with a ship-based vibrocorer system using a steel tube with inner plastic liners of 5 m length and 10 cm inner diameter. Liners and aluminium tubes were cut on site into 1.0 to 1.2 m long sections, sealed and stored in a cold room. Before laboratory analyses, they were split longitudinally, recorded and macroscopically described (Bulian et al., Reference Bulian, Enters, Schlütz, Scheder, Blume, Zolitschka and Bittmann2019; Elschner et al., Reference Elschner, Scheder, Bungenstock, Bartholomä, Becker, Capperucci, Enters, Schlütz, Wehrmann and Hoffmann2021).
Of 160 cores collected by the WASA team, 37 contained peat layers. These were mostly from the back-barrier tidal flats of Norderney, four of them northwest of the island. They reached from about +1 m NHN (German ordnance datum) down to about −16 m NHN. In seven cores a second peat was intercalated in lagoonal, or on top of lagoonal sediments overlain by marine deposits. About 180 slices, each 1–2 cm thick, were taken from the cores for dating and palaeoecological analyses mainly from the peats, some from lagoonal sediments and occasionally from the underlying sands including tree roots. About 130 selected slices were treated in a 10% potassium hydroxide (KOH) solution and were wet-sieved on a 63 µm mesh, their botanical (and zoological) remains handpicked and identified using reference collections of the NIhK and literature sources (e.g. Grosse-Brauckmann, Reference Grosse-Brauckmann1964, Reference Grosse-Brauckmann1972, Reference Grosse-Brauckmann1974; Körber-Grohne, Reference Körber-Grohne1967; Behre, Reference Behre1976; Grosse-Brauckmann & Streitz, Reference Grosse-Brauckmann and Streitz1992). If seeds of rushes were numerous, about 20 randomly picked seeds were identified in detail (Körber-Grohne, Reference Körber-Grohne1964).
One hundred selected botanical fractions were AMS 14C-dated. The dated horizons concentrate on the start of marine influence, so the top of the basal peats and the base of the overlying lagoonal sediments as well as the intercalated peats were dated. For dating, the botanical remains of a sample were divided into up to eight species-specific fractions, depending on the amount of material and state of preservation (waterlogged, charred). Fruits of Cladium mariscus (fen sedge) and sclerenchyma spindles of Eriophorum vaginatum (cotton grass) in particular yielded enough material for dates of single species, as well as alder wood and sclerotia of Cenococcum geophilum. Also, waterlogged or charred twigs and leaves of Calluna vulgaris (common heather) and Erica tetralix (bell heather) were used, either separately or together. Where necessary, assemblages of taxa, charred stems mostly of grasses or the residual <63 µm bulk fine fraction, cleaned in KOH, were used. In case of material shortage, two adjacent samples were combined and treated together. To test the reliability of the various materials, additional fractions (charred, uncharred, different taxa etc.) were dated in parallel.
The dating was done at the Poznań Radiocarbon Laboratory (Goslar & Czernik, Reference Goslar and Czernik2000). Calibration was done with OxCal v. 4.3 (Bronk Ramsey, Reference Bronk Ramsey2009) including deposition models (Bronk Ramsey, Reference Bronk Ramsey2008; Bronk Ramsey & Lee, Reference Bronk Ramsey and Lee2013). Combined ages were calculated for all parallel fractions of potentially the same context, but disregarded if the ages of the fractions were not similar within the 95% probability of the OxCal χ2 test. Data in the text refer to mean ages with 2σ intervals (Bronk Ramsey, Reference Bronk Ramsey2009).
The description of the successive landscape stages in the area around Norderney is based on the temporary and spatial past occurrences of ecologically indicative plant groups. To discover the influence of salinity, we mapped the appearance of fruits and seeds of plants characteristic of salt meadows and salt marshes like Aster tripolium, Triglochin maritima (sea arrowgrass), Juncus gerardii and Salicornia europaea, in addition to linings of foraminifers (Shumilovskikh et al., Reference Shumilovskikh, Seeliger, Feuser, Novenko, Schlütz, Pint, Pirson and Brückner2016). We also applied the Ellenberg indicator values (EIV) (Ellenberg et al., Reference Ellenberg, Weber, Düll, Wirth and Werner2001; Leuschner & Ellenberg, Reference Leuschner and Ellenberg2017), an approach also often used by archaeobotanists (e.g. Willerding, Reference Willerding1979; Hill, Reference Hill1999; Rösch et al., Reference Rösch, Ehrmann, Herrmann, Schulz, Bogenrieder, Goldammer, Hall, Page and Schier2002; Schepers et al., Reference Schepers, Scheepens, Cappers, van Tongeren, Raemaekers and Bekker2013; Bogaard et al., Reference Bogaard, Hodgson, Nitsch, Jones, Styring, Diffey, Pouncett, Herbig, Charles, Ertuğ, Tugay, Filipovic and Fraser2016) to identify differences in soil quality. For the most reliable results when using this actualistic approach, we have considered plants with clear ecological needs (EIV 8 and EIV 9) and therefore low synecological variance. Moreover, we use indicator species, as phytosociological syntaxa may have changed over time and modern analogues may be sparse or missing.
Results
The peat-bearing cores generally consisted, from bottom to top, of Pleistocene Geest sands with some early Holocene modifications, followed by basal peats covered by lagoon and marine deposits and locally intercalated peats. Seeds, fruits and vegetative plant remains of about 125 taxa were identified, when possible to species level (see table in the Supplementary Material available online at https://doi.org/10.1017/njg.2021.4). The locations of all cores containing peat are shown in Figure 1. Based on the dates, sediment and peat analyses, the most widespread habitats in the buried former wetlands of the present-day Wadden Sea area around Norderney are reconstructed in the following subsections, including aspects of dynamic landscape changes.

Fig. 1. Positions of cores with basal peats (white circles) and cores with basal and intercalated peats (black). Tidal flats (Watt) in green, islands in yellow, diked hinterland in the southeast. Depth counters given for north of the islands. Here and in some of the following maps overlapping core symbols are rearranged for clarification; the transect given in Figure 4 is shown as a stippled line.
Before arrival of the sea
With the exception of a few charred remains, the vegetation before the start of deposition of the basal peats is hardly preserved at all. Charred twigs and leaves of Erica tetralix and Calluna vulgaris from the top of the sand of the former Geest at the bottom of the basal peat layer dated to 8750–8450 cal BP (core VVC15) and 8980–8580 cal BP (core W3). They formed here a few centuries after the beginning of the middle Holocene in the Atlantic period, which started at around 9200 cal BP (Barckhausen & Müller, Reference Barckhausen and Müller1984; Dörfler et al., Reference Dörfler, Feeser, van den Bogaard, Dreibrodt, Erlenkeuser, Kleinmann, Merkt and Wiethold2012), and a several hundred years after their early Boreal spread on the Geest slopes around −30 m NHN at about 10,700 cal BP (Wolters et al., Reference Wolters, Zeiler and Bungenstock2010). The early Holocene is represented only by a tiny, unidentified charcoal fragment dated to around 9700–9520 cal BP (core N71).
The records of E. tetralix and C. vulgaris point to the establishment of a wet heath dominated by E. tetralix on the Pleistocene sands, at the latest after the climatic amelioration of the Atlantic period, and long before any pronounced human influence. This predates by about three millennia the formation of wet heath as previously suggested by Menke (Reference Menke1963) and underlines its natural origin that has long been debated (Firbas, Reference Firbas1937; Tüxen, Reference Tüxen, Lauer and Klink1978). As some of these wet heath communities did not include Sphagnum mosses (Leuschner & Ellenberg, Reference Leuschner and Ellenberg2017), they did not form peat archives of this early phase of landscape development. In contrast to this Atlantic wet heath, the Calluna-dominated dry inland heathlands with Juniperus communis (juniper) result from human activities (Menke, Reference Menke1963; Behre, Reference Behre1999b; Karg, Reference Karg2008).
On lower-situated slopes of the former Geest, as proved by Grohne (Reference Grohne1957) for the regions around the recent islands of Juist and Baltrum, the peats are older and E. tetralix has grown here since the early Holocene (Preboreal), forming a kind of Sphagnum-rich wet heath in the Boreal at the latest. In higher places around Juist and Baltrum, E. tetralix was not found before the Atlantic period either, and C. vulgaris dominated on the sands together with Sphagnum spp. but without Juniperus (Grohne, Reference Grohne1957). The vegetation fixed the unstable Geest surface, and recognisable input of aeolian material like sand and dust ceased, but only by the beginning of the Atlantic period (Grohne, Reference Grohne1958) or, as demonstrated by sand layers in core W1, even after 6500 cal BP. Lake sediments as described for the Preboreal and the preceding late glacial Younger Dryas period (Grohne, Reference Grohne1957, Reference Grohne1958) were not recognised.
A fringe of alder carrs facing the rising sea
In pollen records, the strong increase of Alnus around 9200 cal BP usually marks the change from the Boreal to the warmer Atlantic period (Barckhausen & Müller, Reference Barckhausen and Müller1984; Dörfler et al., Reference Dörfler, Feeser, van den Bogaard, Dreibrodt, Erlenkeuser, Kleinmann, Merkt and Wiethold2012). Our oldest records of alder, from site N57 at a depth of −16.6 m NHN, date to 7930 cal BP and are therefore much younger. Core N57 also marks the earliest peat accumulation in our data set, starting probably several hundred years before the top of the 42 cm thick peat layer was formed. Further samples, one more with alder fruits and three samples of alder roots, tend to be younger with increasing elevation towards the Geest (Fig. 2).
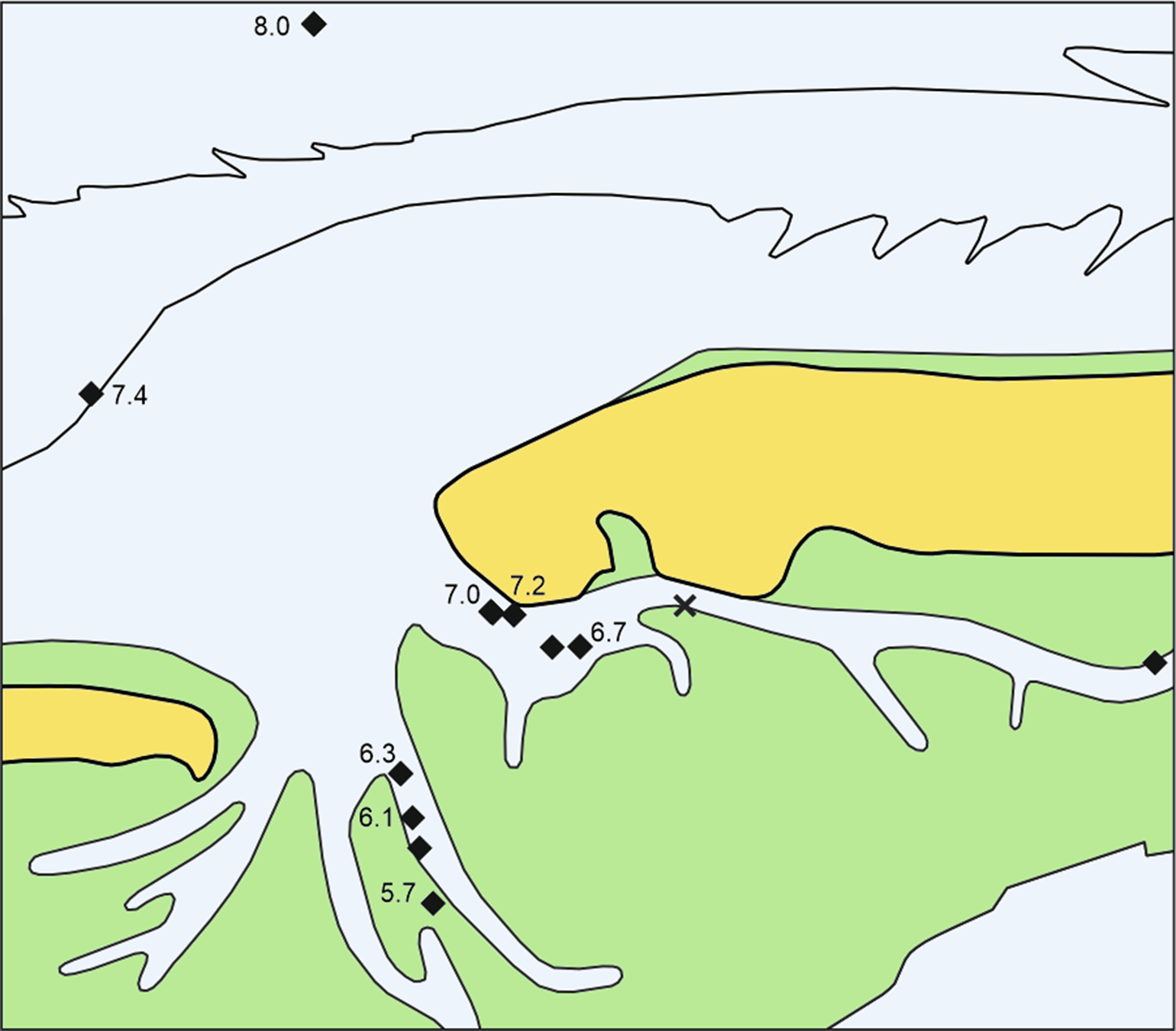
Fig. 2. Sites with alder carr peat and ages of dated Alnus remains in ka cal BP. X = tree roots in the Geest sand without basal peat (core N27). For explanations, see Figure 1. In place of 8.0 read 7.9 here and hereinafter.
Cenococcum geophilum is a mycorrhizal fungus on tree roots which forms sclerotia, lumps of hardened mycelium. Such sclerotia from the top of the peat in core N71 and alder roots from the underlying sand are of the same age according to the χ2 test. The occurrence of sclerotia at the top of the peat shows that this combined age of around 7190 cal BP probably represents the last trees there. Cenococcum sclerotia near the bottom of this peat dated to 7270 cal BP point to tree growth about 100 years earlier, but it is uncertain if the trees were there earlier. This phase of peat with alder and sclerotia as recorded in the present study lasted until about 5700 BP. For practical reasons of landscape reconstruction, the dates of alder roots from sands in this study are related to the middle level of the overlying basal peats, in case of core N71 to the level with the Cenococcum sclerotia of the same age. As no wood of other trees appeared in those samples, Cenococcum was most probably growing on the alder roots.
Taking the dated uppermost peat layers as sea inundation marker points, the flooding of the basal carr peat reflects an increasingly slower sea-level rise. Estimates for peat compression vary widely from, for instance, 25% (Linke, Reference Linke1979), 40% in a few centuries (van Asselen et al., Reference van Asselen, Stouthamer and Smith2010) up to 80–90% (Bennema et al., Reference Bennema, Geuze, Smits and Wiggers1954). If the compaction was 50%, the rate of sea-level rise would have decreased nearly by a factor of ten, from about 10.1 mm a−1 around 8 ka ago, to about 1.1 mm a−1 around 6 ka (Table 1). The initially rapid rise in sea level, its quick slowing down and the height of the reconstructed sea level are in good agreement with earlier results from the southern North Sea (Behre, Reference Behre2007; Bungenstock and Schäfer, Reference Bungenstock and Schäfer2009; Meijles et al., Reference Meijles, Kiden, Streurman, van der Plicht, Vos, Gehrels and Kopp2018) (Fig. 3).
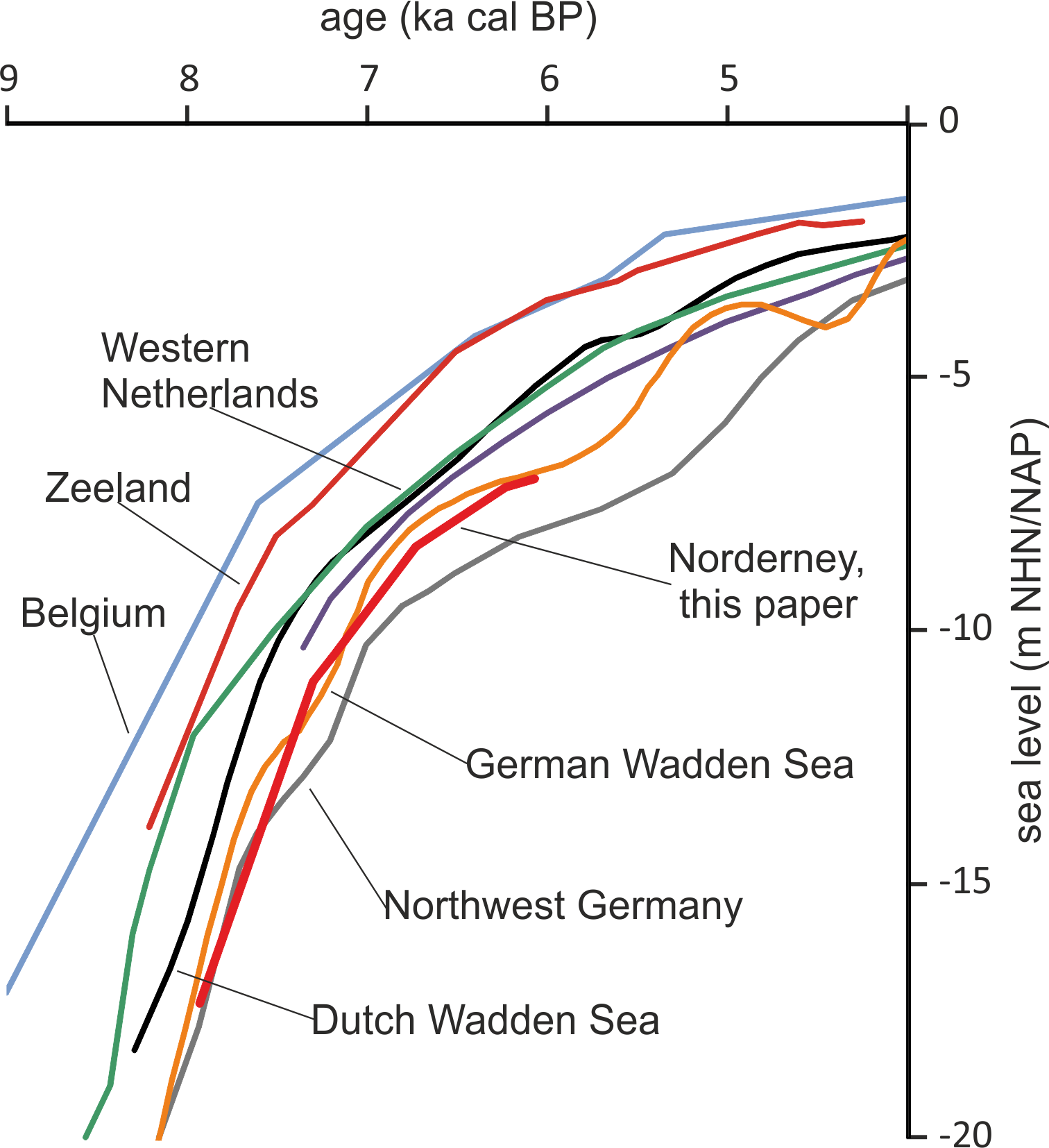
Fig. 3. Early sea-level change at Norderney based on alder carr flooding (this paper) in comparison with reconstructions from other sources and interpretations mostly following Meijles et al. (Reference Meijles, Kiden, Streurman, van der Plicht, Vos, Gehrels and Kopp2018). Other sources: Belgium (Denys and Baeteman, Reference Denys and Baeteman1995), Zeeland, Netherlands (Kiden, Reference Kiden1995; Vink et al., Reference Vink, Steffen, Reinhardt and Kaufmann2007), Western Netherlands (Hijma & Cohen, Reference Hijma and Cohen2010), Dutch Wadden Sea (Meijles et al., Reference Meijles, Kiden, Streurman, van der Plicht, Vos, Gehrels and Kopp2018), Northwest Germany (Vink et al., Reference Vink, Steffen, Reinhardt and Kaufmann2007), German Wadden Sea (Behre, Reference Behre2007).
The dates from the bulk fine material of sand layers below the basal peats probably show the beginning of accumulation and preservation of plant material, most probably roots of wet heath plants, due to the start of waterlogged conditions from increasing soil moisture at a given site. This indicates groundwater levels near the sand surface and predates the later groundwater discharge and start of peat accumulation by an unknown but most probably short time interval. This indirect influence of the sea level took place about 500 (490, >570, 600) or even 900 years before a site was inundated. The duration of this period in core N78 seems to be much overestimated, due to dating charred material which probably derived from an earlier stage of landscape development.
Table 1. Cores with dated alder carr including ages, depths and calculated rates of sea-level change. To compensate for an estimated compression of 50% (see text) the heights of the tops of the carr peats were increased by adding the peat thickness (1). For integration with Figure 4 the recent MTHW height of about 1.13 m above NHN for Norderney was subtracted (Behre, Reference Behre2003), uncertain data in italics.
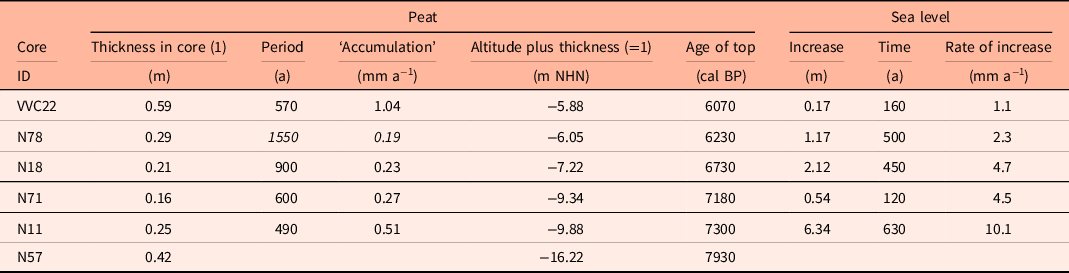
Considering the initial rapid sea-level rise, the interval of organic accumulation at site N11 points to a sea level about 5 m below the later carr site, when paludification started. Here the swampy fringe facing the sea was about 3 km wide at the beginning. These magnitudes may also hold true for the lower parts of the Geest slope which are not included in the present study. The marsh formation which followed at site N71 started shortly afterwards. Due to the gentle Geestslope north of site N71, the swampy fringe doubled in width (Fig. 4).
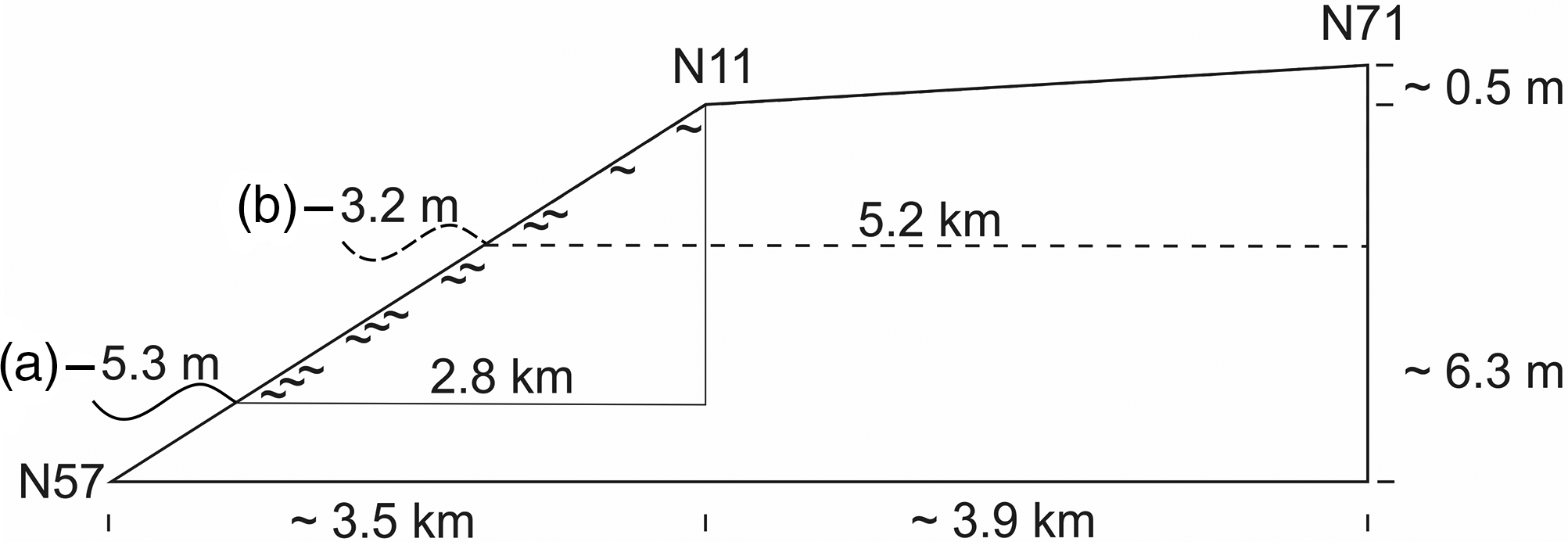
Fig. 4. Diagram showing the approximate relative altitudinal position of the sea level and the width of the zone with groundwater discharge around the time when paludification (∼) started at (a) core N11 and additionally for (b) core N71. The height differences between the tops of the carr peat and the contemporary sea level are given in Table 1; approximate distances between core sites and the (former) coast along the transect are shown in Figure 1.
The situation changed considerably when the sea, by then only slowly rising, flowed into the flat today back-barrier area about 6500 years ago. The alder carr at site VVC22 started to grow when the sea level was already close to its own level. Here, the rate of peat accumulation kept up with the average sea-level rise for some time (Behre & Streif, Reference Behre and Streif1980; Streif, Reference Streif1982). This implies a situation of very strong freshwater discharge which prevented permeation by salt water. Possibly the site received extra fresh water through a channel running across the former Geest (Streif, Reference Streif1990). This could also explain a peat thickness twice as high compared to carrs located lower on the steep slope and the occurrence of the second thickest carr peat within the same channel (core N108, 43 cm). The rising sea may have created a situation like an estuary. Roots of alder in the Geest sand at −7.9 m NHN indicate an older carr nearby in a lower-situated channel to the east (core N27).
As the sea level rose, so did the local drainage system and the associated groundwater table, until freshwater discharge enabled carr development on the Geest slope. With this strong causal link to sea-level rise, the carr peats in our investigation area can be classified as Basistorfe (lower peats) in terms of Lange & Menke (Reference Lange and Menke1967). As macro remains demonstrate, the widespread alder was accompanied by Betula (birch) and also Pinus (pine). Quercus (oak) grew probably together with Corylus (hazel) on drier sites on higher land above the wet carrs (Behre, Reference Behre2004), but they were found only sporadically (see Supplementary Material available online at https://doi.org/10.1017/njg.2021.4).
Halophytes after the alder carrs and later
With the increasing marine influence from the approaching sea, typical plants of salt meadows spread. Fruits and seeds of the halophytes Aster tripolium, Triglochin maritima and Salicornia europaea occurred in several samples and Ruppia maritima in one. S. europaea is an annual pioneer on mud and sand especially of the intertidal zone, while A. tripolium and T. maritima are also typical plants there and on salt meadows at higher elevations (Fig. 5). R. maritima grows, for instance, in brackish pools (Clapham et al., Reference Clapham, Pearsall and Richards1942; Davy & Bishop, Reference Davy and Bishop1991; Dijkema, Reference Dijkema1994; Davy et al., Reference Davy, Bishop and Costa2001; Freund et al., Reference Freund, Gerdes, Streif, Dellwig and Watermann2004). These species, with indicator values (EIV) for salt of 8 to 9, indicate sites with the highest salinity (Leuschner & Ellenberg, Reference Leuschner and Ellenberg2017).
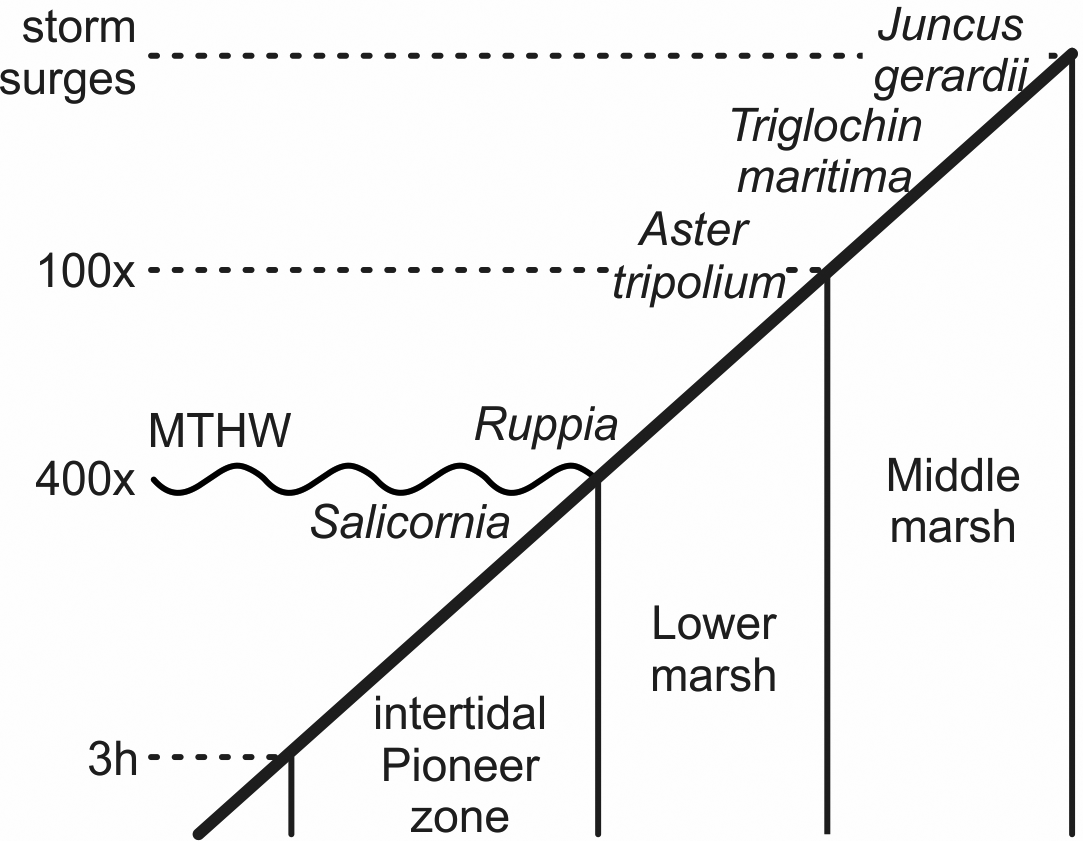
Fig. 5. Schematic zonation of the tidal marshes and times of inundation (h, hours per tide; x, tides per year; there are two tides a day). Salicornia europaea grows around the mean tide high water (MTHW) and below it to where the tidal flat is inundated up to about 3 hours per tide. Ruppia maritima grows in small brackish pools, Aster tripolium in the frequently flooded lower marsh area. Triglochin maritima and Juncus gerardii grow up to where regular storm surges commonly reach (after Erchinger, Reference Erchinger1986; Streif, Reference Streif1990; Vos & Gerrets, Reference Vos and Gerrets2005).
The first occurrences of these halophytes are noted at the lowest level (core N77) at around 7600 cal BP (Fig. 6). The next finds are from further up the Geest slope and with decreasing ages with height. From our dates, sites came under salt influence a few hundred years after the first occurrence of trees. Some halophytes may have been growing directly on the seawater-soaked peats, as can be observed today at Jadebusen near Sehestedt. This rapid succession and the associated gradient to younger ages going up the Geest show the strict relationship of alder carr formation with rising sea level, not only at its beginning but also its end.

Fig. 6. Salt influence. Sites with halophytic herbs, Aster tripolium, Triglochin maritima, Salicornia europaea and Ruppia maritima (red filled circles), linings of foraminifers (blue dots) and seeds of Juncus gerardii (Jg) and J. subnodulosus (Js). Sample ages in ka cal BP, for halophytic herbs underlined, for foraminifers with an asterisk; Jg and Js follow the sample age if they are present. The red line denotes the border between the dominances of J. gerardii and J. subnodulosus; its lobe to the south follows the palaeochannel of the Hilgenrieder Rinne (Streif, Reference Streif1990). For explanations, see Figure 1.
As indicated by the wide distribution of halophytic herbs, the development of mud flats and salt meadows exceeded the area of alder carr in the flat back-barrier area but remained restricted to south of the tidal channel between Norderney and the island of Juist, and was absent from the eastern back-barrier area. Nevertheless, linings of foraminifers in the top layers of the peat indicate marine influence in the eastern part of the study area already around 5.5 ka, which is long before the overall disappearance of the halophytic herbs. Obviously, time and salt did not limit the halophyte expansion but probably the geomorphological conditions as discussed below.
As well as these herbs, the salt-tolerant Juncus gerardii (EIV S7) also occurred, which is a typical perennial rush on the upper parts of the salt meadows. Despite an expected extensive spread of the small and light seeds (0.55–0.75 mm) by water, wind or birds, their distribution is quite similar to that of the halophytic herbs. The diaspores (seeds, fruits) of all these five halophytes consistently reflect the former distribution of salt meadows with intertidal salt marshes closer to the sea. South of the area with J. gerardii and at probably slightly higher elevations, the less salt-tolerant J. subnodulosus (EIV S2) indicates less marine-influenced habitats.
J. gerardii appeared in the eastern back-barrier area (core N108, 6090 cal BP, core VVC9, 5500 cal BP) accompanied by Atriplex (saltbush, orache) and Chenopodium (goosefoot), showing the existence of salt-tolerant vegetation which grew on top of the basal peats (core N107, core N108), as well as brackish lagoonal conditions before flooding by seawater (core VVC9). Today, Atriplex littoralis and A. hastata (A. littoralis/hastata type; Körber-Grohne, Reference Körber-Grohne1967; Behre, Reference Behre1976) grow along drift lines. Together with other Chenopodiaceae, they may have grown together with J. gerardii at higher levels that were reached by seawater only sporadically during spring tides or storm surges, possibly on small erosional cliffs. The absence of frequently inundated stable slopes may have limited the expansion of the salt meadow herbs Aster tripolium and Triglochin maritima to the east. At several sites south of the area with J. gerardii, seeds were found of J. subnodulosus, indicative of less salt-influenced but moist conditions with percolating water, as in fens or communities of large sedges. This may again be evidence for the importance of groundwater from the Geest in opposing marine influence, for instance from occasional storm surges. At some sites J. subnodulosus is followed after a few centuries by J. gerardii (cores N49, VVC9) and herbaceous halophytes (core N49), reflecting a succession driven by a rising sea level. Within the wide distribution of species of Juncus (rushes), the short wings were found from individuals of the rush-feeding plant hopper Conomelus anceps (Delphacidae) (van Geel et al., Reference van Geel, Hallewas and Pals1983), the only lowland representative of the genus (Kunz et al., Reference Kunz, Nickel and Niedringhaus2011).
Phragmites australis and the question of brackish lagoons
Phragmites australis (common reed) occurred in nearly every microscopically analysed core, mostly represented by rhizome fragments occasionally accompanied by fruits. Its well-preserved rhizomes can easily be identified with the naked eye and are one of the most commonly mentioned botanical remains in core descriptions. The perennial grass can form over 3 m high, nearly monospecific stands with dense rhizome masses in the ground up to several decimetres thick. Common reed is widespread in shallow freshwater habitats like lake shores, swamps and carrs as well as in the saline conditions of saltmarshes and brackish lagoons (Packer et al., Reference Packer, Meyerson, Skálová, Pyšek and Kueffer2017). In contrast to its wide former distribution range (Streif, Reference Streif1990), it is rather restricted along the diked coast today. Under experimental conditions, Phragmites benefits from some salt but reacts to near-marine salt concentrations with plant heights of less than 0.5 m (Achenbach et al., Reference Achenbach, Eller, Nguyen and Brix2013). Populations of common reed in Jadebusen on the southern North Sea coast are found at present on soils with a maximum salinity of nearly a third of that of seawater (Scheer, Reference Scheer1953). It is expected to have grown in the former Wadden Sea in brackish conditions of nearly half sea-water salinity (Gerdes et al., Reference Gerdes, Petzelberger, Scholz-Böttcher and Streif2003), which is the limit for the start of dieback on the coast of Denmark today (Lissner & Schierup, Reference Lissner and Schierup1997). From North America, Phragmites is reported from soil salinities of about nearly two-thirds of that of seawater (Burdick et al., Reference Burdick, Buchsbaum and Holt2001). When salinity decreases from reduced influence of tides, reed populations can spread vegetatively by up to 1 m a−1 into adjacent salt meadows (Burdick et al., Reference Burdick, Buchsbaum and Holt2001). The establishment of new stands from fruits or rhizome fragments seems to be largely restricted to salinities below one-third of seawater (Scheer, Reference Scheer1953; Chambers et al., Reference Chambers, Osgood, Bart and Montalto2003). In the widely diked brackish Jadebusen the few observed stands of P. australis were found from +0.7 m to −0.3 m MTHW (mean tide high water) (Scheer, Reference Scheer1953).
As reed rhizomes can penetrate downwards into peat, they may be younger than the surrounding peat or sediment (Streif, Reference Streif1971; Törnqvist et al., Reference Törnqvist, de Jong, Oosterbaan and van der Borg1992). To face this problem, additional fractions like charred stalk remains (probably grasses) and waterlogged leaf fragments of the Thelypteris palustris (marsh fern) were dated as well as some of the waterlogged rhizomes. Except for a c.300 years older charred control specimen, the others gave statistically robust combined ages from the X2 test. The remaining single P. australis ages fit into the time successions of the related depositional sequences. A reason for this generally good agreement might be the relatively high accumulation rate of P. australis peat at about 1 mm a−1 (Törnqvist et al., Reference Törnqvist, de Jong, Oosterbaan and van der Borg1992). Nevertheless, caution is needed and ages of other fractions of peats and sediments might be older than the rhizomes (Sindowski, Reference Sindowski1979). It is important to note that the starting point of a rhizome runner is rarely detectable in a core and P. australis is recognised by some authors as a secondary constituent of peat (Grosse-Brauckmann, Reference Grosse-Brauckmann1972).
The oldest reed beds are reflected by rhizomes and fruits in the carr peat of core N57 (−16.6 m NHN, 7930 cal BP). Younger P. australis finds from higher up on the Geest are directly dated rhizomes from the end of the formation of local basal peat (core N85, −10.3 m NHN, 7140 cal BP; core N84, −9.9 m NHN, 7060 cal BP; core N49, −4.3 m NHN, 4750 cal BP). P. australis rhizomes from the top of lagoonal sediments mark the end of brackish conditions caused by increased marine influence and date to 7620 cal BP (core N57, −16.3 m NHN), 5500 cal BP (core VVC09, −5.8 m NHN) and 3560 cal BP (core N44, −2.9 m NHN). The marine transgression was not a continuous process, but was interrupted by freshwater phases shown by fen and wood peats with P. australis rhizomes at their base at around 6500 cal BP (core N18, core N71) and 4200 cal BP (core N49). The youngest peat with reed remains is about 2900 years old (core N52).
Over a long timespan and in a wide ecological range from freshwater fens, swamps and carrs to brackish lagoons, Phragmites reed beds were a long-lasting part of the landscape in various appearances from about 7950 to 2850 cal BP.
Despite the common macroscopic and microscopic records of P. australis rhizomes, the ecological significance of their occurrence and the dates from the rhizomes are ambiguous. Above-ground remains of ecologically more restricted plants are better suited for dating and environmental reconstructions.
Fertile soils and freshwater conditions
Besides salinity, the availability of nutrients and the soil pH, particularly the base status, are important ecological factors. As demonstrated by the presence of Erica and Calluna, the original sandy soils of the Geest slopes were acidic and barren. The available substrate qualities changed radically with the rising sea. Since the earliest alder carr formation (core N57), nitrophilous aquatic plants such as Alisma plantago-aquatica (water-plantain) and the swamp plants Eupatorium cannabinum (hemp agrimony) and Sonchus oleraceus (common sow thistle) occurred, indicating the release of nutrients by decay of the previously accumulated organic material. With the accumulation of carr and fen peat caused by the rising sea level, a nutrient-rich fringe of vegetation with highly nutrient-demanding plants (Table 2) moved up the Geest slope (Fig. 7). The occurrence of nitrophilous Atriplex littoralis/hastate type indicates fertile marine substrates in connection with lagoonal conditions. Sources for the nutrients would have been, for instance, redeposited peat, seaweed as well as animal corpses and faeces (Liebezeit et al., Reference Liebezeit, Wöstmann and Wolters2008).
Table 2. Ellenberg indicator values (EIVs) of taxa found in the WASA cores with high requirements for nutrients (EIV N 8–9), bases (EIV R 8–9) or salt tolerance (EIV S 8–9), and information about their annual (a) or perennial (p) life forms (Ellenberg & Leuschner, Reference Ellenberg and Leuschner2010). EIVs vary or are unknown within the species aggregate Salicornia europaea; defined salt values range from 7 to 9


Fig. 7. Records of nitrophilous plant taxa (EIV N 8–9, green circles) with oldest ages (ka cal BP). All sites with seeds of any Juncus species (X) and with Conomelus anceps wings (white dots) are marked. For explanations, see Figure 1.
The previously mentioned nitrophilous Sonchus oleraceus requires a high base supply as well as high nitrogen and is, alongwith Berula erecta (lesser water-parsnip), one of the earliest species in this indicator group to appear, both found in the oldest carr peat (core N57) at latest around 7930 years ago (Fig. 8). As the Geest sands would have been poor in carbonates and other basic compounds, extra sources must be considered. Possibly the groundwater feeding the carr carried base-rich minerals from the Lauenburg clay, which contains 10% carbonate (Meyer, Reference Meyer2017) or the more widespread glacial tills of the Geest. Both sources exist below the western area of Norderney at about −10 m NHN (Streif, Reference Streif1990), from where the ancient channel of the Hilgenrieder Rinne passes towards site N57 (Sindowski, Reference Sindowski1973). The geological situation may be the reason why no calcicole plants were found from the higher and more westerly sites (core N11, core N84 and core N85). Within the today tidal-flat back-barrier area, base-demanding plants spread soon after about 7000 cal BP, with the latest occurrences at 2900 cal BP, which is contemporary with slowly rising to stationary or even falling sea level, but increased tidal ranges (Franken, Reference Franken1987), together offering sites rich in carbonates. As most plants are intolerant of salt, the calcareous marine sediments must have been fed by precipitation or fresh groundwater.

Fig. 8. Calcareous soils, freshwater swamps, lakes and raised Sphagnum bogs. Finds of calcicole plant taxa (EIV R 8–9, blue filled circles), Cladium mariscus (X), freshwater bryozoans (black dots) and Sphagnum bogs (green diamonds). Ages in ka cal BP, occurrences of calcicole plants shown in blue italics, C. mariscus underlined, bryozoans asterisked, and tops of the eroded Sphagnum peats in green. For explanations, see Figure 1.
Cladium mariscus (fen sedge, Fig. 8) depends strongly on calcareous substrates and is strictly limited to fresh water (Conway, Reference Conway1942; Hill, Reference Hill1999; Leuschner & Ellenberg, Reference Leuschner and Ellenberg2017). It may have dominated, or formed together with Phragmites australis, dense reed and sedge beds along lake shores, also including Sparganium erectum, Typha sp. and Alisma plantago-aquatica. In swampy areas and fens, Cladium could have grown together with Hydrocotyle vulgaris, Lythrum salicaria, Filipendula ulmaria (meadowsweet), Eupatorium cannabinum and J. subnodulosus, which were also found (Conway, Reference Conway1942; Garve, Reference Garve2007). The existence of freshwater lakes or ponds is shown by numerous statoblasts of freshwater bryozoans (Wood, Reference Wood2005; Wöss, Reference Wöss2005). Cladium is nowadays restricted to small spots on the westernmost East Frisian Island of Borkum and the northern Jadebusen near Hooksiel (Bettinger et al., Reference Bettinger, Buttler, Caspari, Klotz, May and Metzing2013). In contrast, this sedge was widespread along the entire German Wadden Sea coast in the past (Grohne, Reference Grohne1957; Wiermann, Reference Wiermann1962; Menke, Reference Menke1968). South of Norderney, stands of this sedge characterised the landscape for over 4000 years (6750–2850 cal BP). Locally, the alder carr already included pools with bryozoans and with increasing wetness turned into Cladium-dominated reed beds.
Climate as a controlling factor?
The climatic needs of some of the more common taxa in terms of minimum average July temperatures are listed in Table 3. As demonstrated by the reconstructions of Zagwijn (Reference Zagwijn1994) for instance, they occur sometime later or even long after their listed temperature requirements would have been reached or already exceeded by the Holocene climate amelioration. Therefore, the sea was the dominating factor for the observed changes in vegetation composition. The same holds true for trees like Alnus (alder), Betula sp. (birch), Pinus sylvestris (pine) and Quercus sp. (oak) as well as Corylus avellana (hazel). They spread in northern Germany (long) before they occupied the newly emerging vegetation belt facing the rising sea at Norderney (Behre, Reference Behre1967; Giesecke et al., Reference Giesecke, Brewer, Finsinger, Leydet and Bradshaw2016, Reference Giesecke, Brewer, Finsinger, Leydet and Bradshaw2017). In contrast, precipitation played an important role. The large amount of precipitation caused swampy conditions and led to the development of carrs on the Geest slopes several metres above the rapidly rising sea level and it then maintained the freshwater conditions inside the back-barrier area, and the moist oceanic climate also led to the formation of raised bogs.
Table 3. The earliest occurrence of selected species in WASA cores and their requirements for minimum middle July temperatures at their recent distribution limits and for middle January and middle July temperatures at their centres of distribution, from probability density function analyses (Brinkkemper et al., Reference Brinkkemper, van Geel and Wiegers1987; Aalbersberg and Litt, Reference Aalbersberg and Litt1998; Kühl et al., Reference Kühl, Gebhardt, Litt and Hense2002)

Podzols before raised Sphagnum bogs on the Geest
Independent of the sea level, but resulting from the high precipitation from an oceanic climate, the wet heath turned into a complex of rain-fed raised Sphagnum bogs on some higher slopes in the south (Fig. 8). The complex of bogs spread up the Geest for some kilometres into the now diked hinterland and about 15 km into the western back-barrier region of the neighbouring island, Juist, where Sphagnum peats have been described by Körber-Grohne (Grohne, Reference Grohne1957, Reference Grohne1958). In some cores, the Pleistocene sands of the Geest are coloured black like the overlying organic material and so caution is needed when identifying the exact start of peat formation (van de Plassche et al., Reference van de Plassche, Makaske, Hoek, Konert and van der Plicht2010). Below this black layer, caused by infiltration of humic colloids, several centimetres of sand was bleached to a lighter greyish colour (as already reported by Grohne, Reference Grohne1957) suggesting the existence of a deep downwards bleached podzolic soil before the start of Sphagnum peat formation. Analyses of the humic layer lying directly on the sand revealed an extraordinarily large number of local and wind-transported pollen grains containing dark-coloured fungal hyphae (Fig. 9). Such fungal decayed pollen is a result of increased fungal metabolic activity during relatively dry phases and was first described by Shumilovskikh et al. (Reference Shumilovskikh, Schlütz, Achterberg, Kvitkina, Bauerochse and Leuschner2015) from several bogs in northern Germany. Sewell (Reference Sewell1959) identified dark-coloured fungal mycelia as a common component in all kinds of investigated heathlands. Therefore, the appearance of such fungal decayed pollen on the Geest sands seems to represent raw humus accumulation on heathland preserved by the subsequent Sphagnum peat formation. Raw humus is a characteristic accumulation on podzols under poorly decomposable heath vegetation. Due to aeration, the layers directly on the sands yielded no well-preserved material. Dates of the sand/humus contact had to be done using charred vegetative remains of Erica tetralix, Calluna vulgaris and Eriophorum vaginatum, and for the sands also with fine bulk organic material. Sphagnum peat dates used the same species but both charred and waterlogged and also as fine bulk material. One date is from sclerotia of C. geophilum, one of the fungi with dark-coloured mycelia (Gadd et al., Reference Gadd, Watkinson and Dyer2007). Paired charred and waterlogged fractions of the bog slices revealed statistically identical ages, indicating continuous Sphagnum peat deposition without significant vertical shift or entry of external material.

Fig. 9. Palynomorphs from core W1, clockwise from upper left. A pollen tetrad of Calluna vulgaris with two compartments filled with dark-coloured fungal hyphae and an infected five-pored pollen grain of alder, infection most probably after transportation to the site by wind (cf. Shumilovskikh et al., Reference Shumilovskikh, Schlütz, Achterberg, Kvitkina, Bauerochse and Leuschner2015). Both from the same wet-heath raw humus layer on top of the Geest sands. Marine non-pollen palynomorphs Micrhystridium (echinate) and Pterosperma (reticulate) as indicators of marine influence in peats (http://nonpollenpalynomorphs.tsu.ru, Bakker & van Smeerdijk, Reference Bakker and van Smeerdijk1982; Tomas & Hasle, Reference Tomas and Hasle1997). Scale bar 10 µm.
The earliest dates from charred plant material from the sands (core W3, 8750 cal BP, core VVC15, 8590 cal BP) indicate a wet heath on the Geest about a millennium before water accumulation by Sphagnum mosses led to the preservation of biological material in the ground (fine bulk material, 7560 cal BP, 7670 cal BP, 7380 cal BP). The stratigraphically lowest dates from a bog peat layer are from its centre and revealed a combined age of 5940 cal BP. The top of the Sphagnum peat from five cores provided up to three parallel dates, such as 5480 cal BP, 5410 cal BP, 5400 cal BP, 5400 cal BP and 5360 cal BP. The peats exhibited an uneven eroded surface, perforated by mussel shells and borings filled up with lagoon sediments containing reworked peat debris, but filled with marine sediments at the lowest site (core VVC15).
Due to its height above the contemporary sea level and peat loss by abrasion, the marine flooding of the bogs most probably happened significantly later than 5400 cal BP. A climatically driven ending of bog growth long before the flooding can be excluded. The bogs further up the Geest kept on growing and new ones even started to develop on the nearby Geest (Grohne, Reference Grohne1957) and in the north German lowland (Petzelberger, Reference Petzelberger2000; Gerdes et al., Reference Gerdes, Petzelberger, Scholz-Böttcher and Streif2003). Moreover, in the whole North Atlantic realm a strong increase in bog formation is reported to have started shortly after about 5400 cal BP (Franzén and Cropp, Reference Franzén and Cropp2007; Kylander et al., Reference Kylander, Martínez-Cortizas, Bindler, Kaal, Sjöström, Hansson, Silva-Sánchez, Greenwood, Gallagher, Rydberg, Mörth and Rauch2018).
Intercalated layers of marine clay in the present bog peats indicate breakages of the peat layers and floating of the top layers during flooding by storm surges, leaving a secondary intercalated clay deposited there, Klappklei (Grohne, Reference Grohne1957; Behre, Reference Behre2004; Bruns et al., Reference Bruns, Bungenstock, Wolters and Freund2015). The marine origin of the clay is shown by characteristic microscopic remains of marine organisms like Pterosperma cf. reticulosa and dinoflagellate cysts (Fig. 9). The breakages of the peat layers are linked to density contrasts and weaknesses of their inner structure, for instance a burnt layer in core W1.
Climate changes at the turn from the Atlantic to the Subboreal (∼5600 cal BP) possibly caused alterations in the Sphagnum species composition and/or a looser peat structure from rapid growth, as seen later at the change from the Subboreal to the Subatlantic (Grohne, Reference Grohne1957; Overbeck, Reference Overbeck1963). A previously reduced peat growth with decomposition could have increased the peat density in favour of later colonisation by mussels. Abrupt changes in the growth of Sphagnum bogs are described as recurrence surfaces in the older literature and the oldest recognised surface is dated by archaeological finds to roughly 5600 cal BP (van Zeist, Reference van Zeist1955).
During storm surges thousands of years later, the upper peat layer could split horizontally along the structural interface and drift away (Behre & Kučan, Reference Behre and Kučan1999). As nearby bog developments were terminated by sea transgression around 2200 cal BP (Grohne, Reference Grohne1957), about 3000 years of Subboreal Sphagnum peat formation was lost by such displacement and abrasion at our study sites, corresponding to about 80 cm of peat in that area (Grohne, Reference Grohne1957). Rhizomes of Phragmites in the upper centimetres of the Sphagnum peat (cores VVC15, W4, W5) indicate that following the removal of peat a fen or lagoonal deposit developed, which is otherwise not preserved in the sedimentary record.
Hunting, fishing, gathering and staying
The changing landscape of the Geest slopes provided over time various habitats with a wide range of opportunities for hunting, fishing and gathering. The coast offered mussels and saltwater fish. The kilometre-wide swampy vegetation fringe with carr provided natural habitats for a variety of animal and plant resources like hazelnuts until around 6 ka. Freshwater fish and waterfowl would have inhabited the then spreading lakes and lagoons. Edible plants like Salicornia europaea, Triglochin maritima or Sonchus oleraceus and others are popular for salads up to the present. The rhizomes of Typha and Alisma plantago-aquatica are a rich source for carbohydrates. The wet heath and bogs offered berries of Empetrum nigrum (black crowberry), Vaccinium oxycoccos (small cranberry) (Grohne, Reference Grohne1957) and possibly other Vaccinium species. Leaves of Mentha aquatica/arvensis (mint) and other Lamiaceae may have been used as herbs. Filipendula ulmaria (meadowsweet) is edible, can sweeten drinks (mead, for instance), hence its common name, and it could have provided a remedy for headaches due to its content of salicylic acid (Behre, Reference Behre1999a; Düll and Kutzelnigg, Reference Düll and Kutzelnigg2016). In addition, other potential drugs and poisons were available such as Solanum nigrum (black nightshade) or Ranunculus sceleratus (celery-leaved buttercup). The vegetation could also have supplied material for making shelters or for firewood and tinder, such as C. mariscus. The cored material, however, did not offer any artefacts or other direct evidence of human activities.
Conclusions
The large number of 14C dates and botanical analyses in the present study illustrates the development of the Wadden Sea around the East Frisian island of Norderney with particular attention to the detailed suitability of the landscape for providing natural resources for early humans. Our reconstructions go further back in time, add many details to earlier reviews (Goldhammer & Karle, Reference Goldhammer and Karle2015; Vos & Knol, Reference Vos and Knol2015) and expand the detailed but spatially restricted analyses of Körber-Grohne (Grohne, Reference Grohne1957, Reference Grohne1958) into the back-barrier region and in the area northwest of Norderney. The sea-level index points and rates of sea-level rise derived from flooded alder carrs are in good agreement with previous results from the southern North Sea (Behre, Reference Behre2007; Meijles et al., Reference Meijles, Kiden, Streurman, van der Plicht, Vos, Gehrels and Kopp2018). With our new data, the sea-level reconstruction of upcoming WASA publications is extended back to around 8000 cal BP and therefore to the inundation of the oldest peat analysed in the project.
An important but so far quite neglected landscape element of the early stage was a kilometre-wide swampy vegetation fringe including alder carr, fed by groundwater discharge from the Geest slopes facing the fast-rising sea. With flooding of the today tidal-flat back-barrier area by a then slowly rising sea and an unchanging or even decreasing marine influence, a vast zone of brackish reed beds and lakes arose. The widespread Cladium mariscus and freshwater bryozoans confirm freshwater conditions. On the higher slopes of the Geest, Sphagnum raised bogs developed under a moist oceanic climate. This highly diverse landscape offered a variety of habitats with animals, fish and numerous wild plants potentially usable by people for various purposes. This hospitable environment disappeared some 2000 years ago (Grohne, Reference Grohne1957), and freshwater conditions ended in the back-barrier area already 3000 years ago. Today, comparable landscape elements still exist, but only in fragments, partly because of the silting-up of the back-barrier areas and partly because of the building of dikes.
Acknowledgements
This study is part of the multidisciplinary project WASA ‘The Wadden Sea as an archive of landscape evolution, climate change and settlement history’ funded by the ‘Niedersächsisches Vorab’ of the VolkswagenStiftung within the funding initiative ‘Küsten und Meeresforschung in Niedersachsen’ of the Ministry of Science and Culture of Lower Saxony, Germany (project VW ZN3197). The authors thank the whole WASA team for the joint work and especially A. Wehrmann and R. Capperucci for drilling, F. Bungenstock and R. Capperucci for the core sketches and Th. Becker for technical support. Thanks go to Th. Goslar for discussion on dating. We thank Wim Hoek and an anonymous reviewer for their profound improvements. Special thanks go to M. Karle for support on getting started and fruitful discussions.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/njg.2021.4.














