Article contents
Immobilization Index of Liquid LLW in Cementitious Grouts
Published online by Cambridge University Press: 15 February 2011
Abstract
The ability of grouts formulated from mixtures of cementitious materials and attapulgite clay to immobilize various chemical species in the projected off-gas waste stream from vitrification of Hanford low level tank wastes was studied. Three different solid blends were evaluated, with cement :fly ash : slag clay weight ratios of 3:3:3:1, 3:0:6:1, and 0:0:9:1. The blended solids were mixed with a simulated low level liquid waste solution containing Na+, NO2-, NO3-, PO43- and OH- ions, in the proportion of 1 liter of solution to 1 kg of solid blend, and were cured either at 22°C (room temperature), 50°C or 90°C. Pore solutions were expressed at various ages and were analyzed to determine the reductions in concentrations of the individual ionic species. The results were expressed in the form of immobilization index (I) calculated for each species. The immobilization indices for Na+ (I Na+ ) and for OH- (IOH-)were similar in each case, and were found to be highest when only slag and clay was present (blend 0:0:9:1). The immobilization index for phosphate, 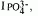 , was 1 in all cases, i.e. phosphate was completely removed from solution. On the other hand removal of NO2- and NO3- ions was generally ineffective.
, was 1 in all cases, i.e. phosphate was completely removed from solution. On the other hand removal of NO2- and NO3- ions was generally ineffective.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1996
References
- 1
- Cited by


