Published online by Cambridge University Press: 20 October 2020
This perspective article summarizes the operational principles of dual-ion batteries and highlights the main issues in the interpretation and reporting of their electrochemical performance.
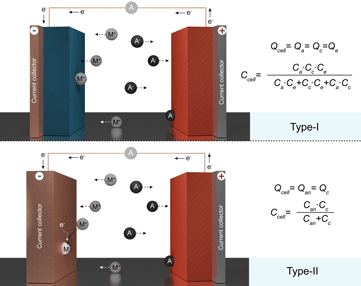
Secondary dual-ion batteries (DIBs) are emerging stationary energy storage systems that have been actively explored in view of their low cost, high energy efficiency, power density, and long cycling life. Nevertheless, a critical assessment of the literature in this field points to numerous inaccuracies and inconsistencies in reported performance, primarily caused by the exclusion of the capacity of used electrolytes and the use of non-charge-balanced batteries. Ultimately, these omissions have a direct impact on the assessment of the energy and power density of DIBs. Aiming to secure further advancement of DIBs, in this work, we critically review current research pursuits and summarize the operational mechanisms of such batteries. The particular focus of this perspective is put on highlighting the main issues in the interpretation and reporting of the electrochemical performance of DIBs. To this end, we survey the prospects of these stationary storage systems, emphasizing the practical hurdles that remain to be addressed.