Article contents
TaWSi amorphous metal thin films: composition tuning to improve thermal stability
Published online by Cambridge University Press: 04 September 2017
Abstract
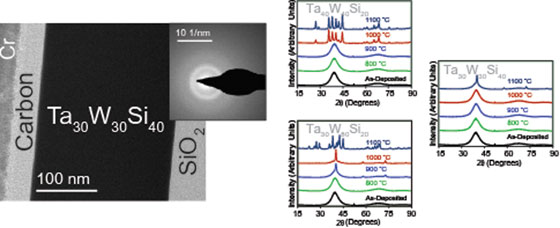
We deposited TaWSi amorphous metal thin films to determine how composition affects film crystallization and oxidation at high temperatures. Films were deposited by magnetron sputtering from targets of nominal compositions Ta : W : Si = 40 : 40 : 20, 30 : 50 : 20, and 30 : 30 : 40, and studied by electron probe microanalysis, electron microscopy, electrical methods, x-ray diffraction, x-ray photoelectron spectroscopy, and atomic-force microscopy. All films remained amorphous to 800 °C or higher temperatures. Films prepared from the target composition 30 : 30 : 40 yielded the film composition Ta41.7W38.4Si19.9, which retained its film integrity and amorphous structure to 1100 °C, even after annealing in air.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
These authors share first authorship.
References
- 7
- Cited by


