Published online by Cambridge University Press: 28 March 2017
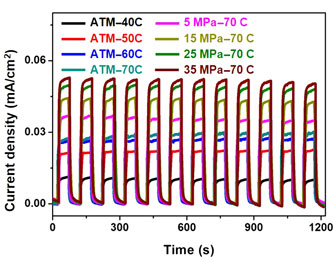
The hydrobaric effect on photoactivity of titanium dioxide (TiO2) fabricated by cathodic deposition in an aqueous solution was evaluated in this study. When the applied pressure was increased to 35 MPa, the water-splitting performance was improved by almost fourfold of the performance of the TiO2 prepared at atmospheric pressure. The surface states effect was significant in the deposited TiO2, which was exploited to affect the charges recombination of TiO2, and thereby enhance the resultant photoelectrochemical water-splitting performance. The hydrobaric cathodic deposition could be extended to fabrication of other metal oxides to eliminate the negative influence from the high-temperature process.