Article contents
Quantitative composition determination by Mössbauer spectroscopy
Published online by Cambridge University Press: 16 December 2019
Abstract
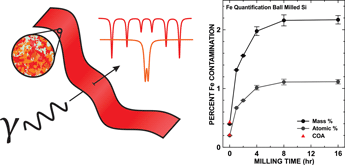
Quantitative Fe content determination of powders by Mössbauer spectroscopy is described. In this method, powder samples and internal standard are combined homogeneously in a plastic film ensuring a thin absorber. This method was verified by quantifying the Fe content of a series of samples and independently confirming by inductively coupled plasma optical emission spectroscopic analysis. Additionally, for the first time, Fe contamination in ball-milled Si as a function of milling time was quantified. It was found that Fe contamination increased with time but surprisingly became steady state at 1.12 ± 0.04 at.% Fe after grain size reduction.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 3
- Cited by



