Article contents
Intracellular microRNA quantification in intact cells: a novel strategy based on reduced graphene oxide-based fluorescence quenching
Published online by Cambridge University Press: 13 July 2018
Abstract
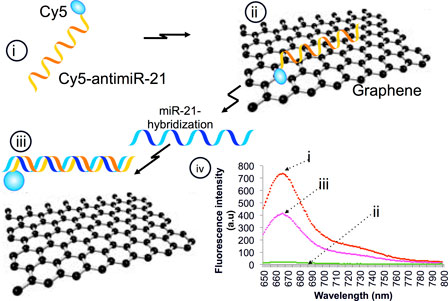
Nanomaterials have been proposed as key components in biosensing, imaging, and drug delivery since they offer distinctive advantages over conventional approaches. The unique chemical and physical properties of graphene make it possible to functionalize and develop protein transducers, therapeutic delivery vehicles, and microbial diagnostics. In this study, we evaluate reduced graphene oxide as a potential nanomaterial for quantification of microRNAs including their structural differentiation in vitro in solution and inside intact cells. Our results provide evidence for the potential use of graphene nanomaterials as a platform for developing devices that can be used for microRNA quantitation as biomarkers for clinical applications.
- Type
- 2D Nanomaterials for Healthcare and Lab-on-a-Chip Devices Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 3
- Cited by



