There is ongoing interest in creating magnets as tiny as possible, to create more efficient hard-drive storage, to perform MRI with injected particles rather than giant rings, and to bring quantum computing closer to reality. As a magnet gets smaller and smaller, it is more likely that interactions with its environment will ruin its internal magnetism. In a recent study in Nature Nanotechnology (doi:10.1038/s41565-018-0296-7), a group of researchers from IBM and several universities reported on the small magnets they made by stabilizing the magnetism of a single atomic nucleus.
Both an electron and a nucleus in an atom have intrinsic angular momentum and can act as tiny magnets. The overall energy of an atom differs slightly depending on whether the electron and the nucleus are magnetically pointing in the same or opposite directions. This energy difference is much smaller than the energy difference between different electron orbits, and the different magnetic states are called “hyperfine structure.” To allow a single atom to act as a magnet, it is necessary to control its hyperfine structure—to dictate the alignment of the electron and nucleus—without having them de-orient due to thermal fluctuations from the environment.
The researchers wanted to stabilize the magnetism of single atoms, and did so using technology developed by IBM in the 1980s. Individual atoms of copper deposited on a magnesium oxide surface on top of silver substrate were used. The researchers modified a scanning tunneling microscope (STM) to emit electrons with a specific magnetic alignment. An electron jumping from the STM tip exerts a torque on an orbital electron in the copper, putting it in a specific spin state. This then dictates the opposite spin state in the nucleus, to conserve overall angular momentum. As the researchers write, “an electron spin flips and a nuclear spin flops,” and this process allows the nuclear spin of atoms to be dictated one at a time.
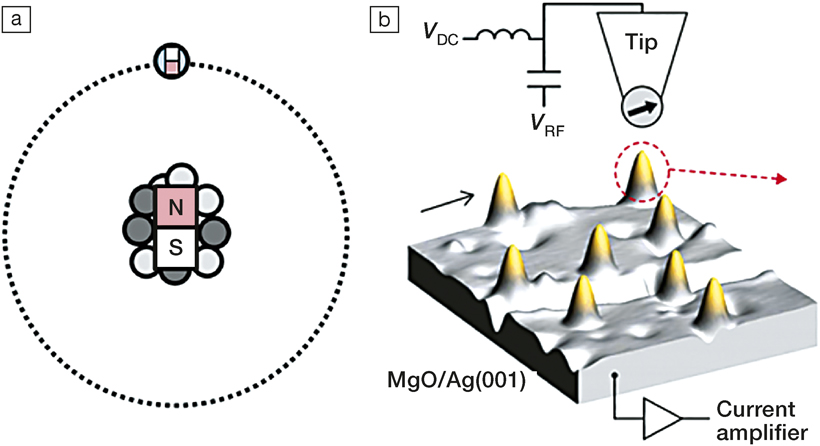
(a) The nucleus of an atom acting as a magnet, with the magnet of the electron pointing in the opposite direction. (b) A scanning tunneling microscope tip interacts with a Cu atom on a surface. Credit: Nature Nanotechnology.
A single-atom magnet is always in a precarious state because the hyperfine energy differences are so small compared to thermal agitation (in this case, electrons scattering from the silver below). The experiment was conducted at a very low temperature (∼1 K) and strong magnetic field (∼1 Tesla) to allow the atoms to retain their hyperfine state without succumbing to environmental perturbations. Even under these conditions, it is expected that fewer than 2% of the atoms would naturally be found in the desired state. Through their STM polarization technique, the researchers were able to improve this by a factor of 17, resulting in 30% of the atoms having the desired nuclear magnetic state.
The researchers are hopeful that in the future this technology can be used to develop spintronics, in which signals are sent by angular momentum rather than charge, and be used for detecting magnetic fields on extremely small scales.


