Published online by Cambridge University Press: 29 November 2013
By virtue of their unique structures, fullerenes exhibit novel chemical transformations. Particularly pertinent to this article are the interesting properties exhibited by fullerenes in the solid state. These molecules are spherical or near-spherical in shape. Molecules with high point-group symmetry, which are not bound strongly in the solid state, tend to crystallize into structures with long-range periodicity of the molecular centers of mass, but the molecular orientations are random or even dynamically disordered. When dynamically disordered, the
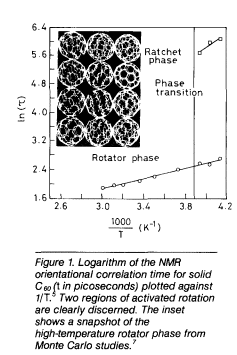
molecules rotate about some preferred axis. C60 and C70 satisfy the criteria for such orientationally disordered solids and exhibit rich phase behavior in the solid state. Since C60 has high electron affinity, it forms anion salts with alkali and alkaline-earth metals as well as with strong organic donor molecules. With tetrakis dimethylaminoethylene (TDAE), which is a very powerful electron donor, C60 forms a 1:1 solid that is ferromagnetic. C60-TDAE is the molecular organic ferromagnet with the highest Tc (of 16 K) known to date. Some of the alkali and alkaline-earth fullerides, on the other hand, show superconductivity, with transition temperatures going up to 33K. We shall briefly examine some of these solid-state properties.