Article contents
New Carbohydrate-Based Polymeric Materials
Published online by Cambridge University Press: 29 November 2013
Extract
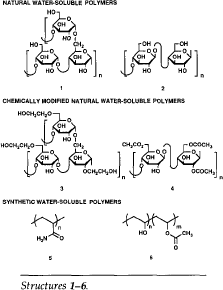
The total world production of water-soluble polymers is estimated to be greater than five million tons per year. Water-soluble polymers are most conveniently described according to their origin in three classes (see Structures 1-6):
∎ Natural polymers, including starch (1) and cellulose (2);
∎ chemically modified natural polymers, including, for example, hydroxyethyl starch (3) and cellulose acetate (4); and
∎ synthetic polymers, the most important of which are polyacrylamide (5) and polyvinyl alcohol (6), (commonly composed of both alcohol and acetate groups as shown). The widespread use of these materials is due to both their availability and the range of useful physical properties found in the various natural and chemically modified natural polymers.
Of the commercial water-soluble polymers, approximately 50–80% are based on natural polysaccharide materials. One of the primary reasons that these materials find such widespread use is the dramatic response of their properties to changes in their functionality and stereochemistry: chemical modification or the combination of polysaccharides with other polymeric materials has yielded materials whose applications range from explosives to food additives. Although efforts directed at controlling the properties of polysaccharides has resulted in a wide variety of useful materials, we felt control of the composition of carbohydrate-based polymers at the molecular level would provide materials with properties superior to those derived from natural and chemically modified polysaccharide materials.
Our approach for the preparation of new carbohydrate-based materials is to use the carbohydrate as a template for the introduction of desired functionality with complete regiochemical and stereochemical control by both chemical and enzymatic methods (Scheme I).
- Type
- Biology and Materials Synthesis
- Information
- Copyright
- Copyright © Materials Research Society 1992
References
- 2
- Cited by


