Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Grguric, B. A.
2002.
Hypogene violarite of exsolution origin from Mount Keith, Western Australia: field evidence for a stable pentlandite–violarite tie line.
Mineralogical Magazine,
Vol. 66,
Issue. 2,
p.
313.
Ping Xu, Zhi
Braterman, Paul
and
Yarberry, Faith
2004.
Handbook of Layered Materials.
Frost, Ray L.
and
Erickson, Kristy L.
2004.
Vibrational spectroscopy of stichtite.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 60,
Issue. 13,
p.
3001.
Génin, Jean-Marie R.
Aïssa, Rabha
Géhin, Antoine
Abdelmoula, Mustapha
Benali, Omar
Ernstsen, Vibeke
Ona-Nguema, Georges
Upadhyay, Chandan
and
Ruby, Christian
2005.
Fougerite and FeII–III hydroxycarbonate green rust; ordering, deprotonation and/or cation substitution; structure of hydrotalcite-like compounds and mythic ferrosic hydroxide.
Solid State Sciences,
Vol. 7,
Issue. 5,
p.
545.
Frost, Ray L.
Erickson, Kristy L.
and
Kloprogge, Theo J.
2005.
Vibrational spectroscopic study of the nitrate containing hydrotalcite mbobomkulite.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 61,
Issue. 13-14,
p.
2919.
Frost, Ray L.
Adebajo, Moses O.
and
Erickson, Kristy L.
2005.
Raman spectroscopy of synthetic and natural iowaite.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 61,
Issue. 4,
p.
613.
Frost, Ray L.
and
Erickson, Kristy L.
2005.
Near-infrared spectroscopy of stitchtite, iowaite, desautelsite and arsenate exchanged takovite and hydrotalcite.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 61,
Issue. 1-2,
p.
51.
Grguric, Ben
and
Riley, Timothy
2005.
Wealth Creation in the Minerals Industry.
p.
311.
Bouzaid, J. M.
Frost, R. L.
Musumeci, A. W.
and
Martens, W. N.
2006.
Thermal decomposition of the synthetic hydrotalcite woodallite.
Journal of Thermal Analysis and Calorimetry,
Vol. 86,
Issue. 3,
p.
745.
Tao, Qi
Reddy, B.J.
He, Hongping
Frost, Ray L.
Yuan, Peng
and
Zhu, Jianxi
2008.
Synthesis and infrared spectroscopic characterization of selected layered double hydroxides containing divalent Ni and Co.
Materials Chemistry and Physics,
Vol. 112,
Issue. 3,
p.
869.
Mills, S. J.
Wilson, S. A.
Dipple, G. M.
and
Raudsepp, M.
2010.
The decomposition of konyaite: importance in CO2fixation in mine tailings.
Mineralogical Magazine,
Vol. 74,
Issue. 5,
p.
903.
Krivovichev, Sergey V.
Yakovenchuk, Victor N.
and
Zhitova, Elena S.
2011.
Minerals as Advanced Materials II.
p.
87.
Mills, S. J.
Christy, A. G.
Génin, J.-M. R.
Kameda, T.
and
Colombo, F.
2012.
Nomenclature of the hydrotalcite supergroup: natural layered double hydroxides.
Mineralogical Magazine,
Vol. 76,
Issue. 5,
p.
1289.
Wilson, Sasha
Harrison, Anna L.
Dipple, Gregory M.
Power, Ian M.
Barker, Shaun L.L.
Ulrich Mayer, K.
Fallon, Stewart J.
Raudsepp, Mati
and
Southam, Gordon
2014.
Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining.
International Journal of Greenhouse Gas Control,
Vol. 25,
Issue. ,
p.
121.
Gole, Martin J.
2014.
Leaching of S, Cu, and Fe from disseminated Ni-(Fe)-(Cu) sulphide ore during serpentinization of dunite host rocks at Mount Keith, Agnew-Wiluna belt, Western Australia.
Mineralium Deposita,
Vol. 49,
Issue. 7,
p.
821.
Kopylova, M.G.
Gaudet, M.
Kostrovitsky, S.I.
Polozov, A.G.
and
Yakovlev, D.A.
2016.
Origin of salts and alkali carbonates in the Udachnaya East kimberlite: Insights from petrography of kimberlite phases and their carbonate and evaporite xenoliths.
Journal of Volcanology and Geothermal Research,
Vol. 327,
Issue. ,
p.
116.
Franchuk, Anatoliy
Lightfoot, Peter C.
and
Kontak, Daniel.J.
2016.
High tenor Ni–PGE sulfide mineralization in the South Manasan ultramafic intrusion, Paleoproterozoic Thompson Nickel Belt, Manitoba, Canada.
Ore Geology Reviews,
Vol. 72,
Issue. ,
p.
434.
Cao, Yaowu
Guo, Qinghai
Shu, Zhu
Zhuang, Yaqin
Yu, Zhengyan
Guo, Wei
Zhang, Canhai
Zhu, Mingcheng
Zhao, Qian
and
Ren, Tianlin
2016.
Application of calcined iowaite in arsenic removal from aqueous solution.
Applied Clay Science,
Vol. 126,
Issue. ,
p.
313.
Melchiorre, Erik B.
Bottrill, Ralph
Huss, Gary R.
and
Lopez, Amanda
2018.
The early Earth geochemical and isotope record of serpentinizing environments from Archean stichtite (Mg6Cr2(OH)16[CO3]·4H2O).
Precambrian Research,
Vol. 310,
Issue. ,
p.
198.
Turvey, Connor C.
Wilson, Sasha
Hamilton, Jessica L.
Tait, Alastair W.
McCutcheon, Jenine
Beinlich, Andreas
Fallon, Stewart J.
Dipple, Gregory M.
and
Southam, Gordon
2018.
Hydrotalcites and hydrated Mg-carbonates as carbon sinks in serpentinite mineral wastes from the Woodsreef chrysotile mine, New South Wales, Australia: Controls on carbonate mineralogy and efficiency of CO2 air capture in mine tailings.
International Journal of Greenhouse Gas Control,
Vol. 79,
Issue. ,
p.
38.
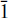 3). These lines were indexed on a hexagonal cell with a = 3.103(2), c = 24.111(24)Å, V = 201.14 Å3 and Z = 3/8. The new mineral is isostructural with the hydrotalcite group and has space group R
3). These lines were indexed on a hexagonal cell with a = 3.103(2), c = 24.111(24)Å, V = 201.14 Å3 and Z = 3/8. The new mineral is isostructural with the hydrotalcite group and has space group R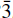 m. The measured density is 2.062 gm/cm3. Woodallite is uniaxial negative with ω = 1.555 and ε = 1.535 (white light); pleochroism is distinct from violet to pinkish lilac. Woodallite forms as a result of hydrothermal alteration of primary magmatic chromite by Clrich solutions at temperatures <320°C. Relict chromite fragments are frequently present in the whorls, and associated magnetite is altered extensively to iowaite. The mineral is named after Roy Woodall, eminent Australian industry geologist.
m. The measured density is 2.062 gm/cm3. Woodallite is uniaxial negative with ω = 1.555 and ε = 1.535 (white light); pleochroism is distinct from violet to pinkish lilac. Woodallite forms as a result of hydrothermal alteration of primary magmatic chromite by Clrich solutions at temperatures <320°C. Relict chromite fragments are frequently present in the whorls, and associated magnetite is altered extensively to iowaite. The mineral is named after Roy Woodall, eminent Australian industry geologist.