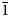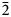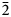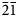Introduction
In 1988, gortdrumite, with the ideal formula (Cu,Fe)6Hg2S5, was described from the Gortdrum mine, Monard, County Tipperary, Ireland (Steed, Reference Steed1983). At that time, its crystal structure remained unknown. During the systematic investigation of ore minerals from the Příbram area, Czech Republic, Škácha and Sejkora (Reference Škácha and Sejkora2013) identified at the Vrančice deposit a mineral having a gortdrumite-like chemical composition, the only difference being represented by the absence of Fe. Indeed, these authors described this phase as an Fe-free gortdrumite-like mineral, with empirical formula (Cu6.26Ag0.01)Σ6.27Hg1.78S4.95. Recently, Bindi et al. (Reference Bindi, Paar and Leblhuber2018) solved the crystal structure of gortdrumite using a sample from Leogang, Salzburg, Austria. It is characterised by an Fe-dominant site, and the chemical formula was revised to Cu24Fe2Hg9S23 (Z = 1). It was then obvious that the mineral from Vrančice is different from gortdrumite. Thus, a crystal-chemical investigation was undertaken, which allowed for the solution of its crystal structure and the proposal of the new mineral vrančiceite.
This new mineral and its name (symbol Vrc) were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2022-114, Sejkora et al., Reference Sejkora, Biagioni, Škácha and Mauro2023). Vrančiceite is named after its type locality, the Vrančice deposit near Příbram, Czech Republic. The holotype material (polished section) is deposited in the mineralogical collection of the Department of Mineralogy and Petrology of the National Museum, Prague, Czech Republic (catalogue number P1P 42/2022); the cotype material is in the collection of the Mining Museum Příbram, Czech Republic (catalogue number 2/2023) and the crystal used for the single-crystal X-ray diffraction study is kept in the mineralogical collection of the Museo di Storia Naturale of the Università di Pisa, Via Roma 79, Calci (PI), Italy, under catalogue number 20024.
Occurrence and mineral description
Occurrence
Vrančiceite was only found in one specimen, 3.5 × 5 × 4 cm in size, collected by one of us (PŠ) in 2012 from the dumps of an old mine exploited in the 16th Century on Vraneč hill, north of Vrančice village (49°37'10.71"N, 14°02'51.69"E), central Bohemia, Czech Republic. This mine exploited the surficial portion of the most important Vrančice vein, Beschert Glück, known as the Pošepný vein from the 19th Century. The last period of mining focused on Ag, Cu, Zn, Pb and U ores from the Alexander shaft from 1947 to 1991; the main Pošepný vein provided up to 95% of production of all the Vrančice deposit (Kopečný, Reference Kopečný2012). The Vrančice deposit belongs to the outer part of the Central Bohemian Plutonic Complex and is located in the endocontact with the rocks of the Barrandien volcano–sedimentary complex. The most common rock cropping out in the area is granodiorite, of a type known as the ‘rim’ type of the Blatná granodiorite (Habásko, Reference Habásko1972). The Vrančice deposit is one of the smaller base-metal and uranium deposits in the outer part of the Příbram ore area.
The old dumps on Vraneč hill are known for the occurrence of >25 minerals (see full list on mindat.org at https://www.mindat.org/loc-236977.html). Vésigniéite was described by Paděra and Johan (Reference Paděra and Johan1957); hedyphane, vanadinite, mottramite, pyromorphite, pseudomalachite, cerussite and iodargyrite were identified by Mrázek and Švihnos (Reference Mrázek and Švihnos1980). Mrázek and Táborský (Reference Mrázek and Táborský1981) found the new mineral čechite, named after Professor František Čech from the Charles University of Prague. The list of minerals from the Vraneč hill was expanded thanks to Mrázek and Švihnos (Reference Mrázek and Švihnos1982) with brandtite, conichalcite, chervetite, volborthite, langite and cinnabar. Mrázek (Reference Mrázek1982) further added descloizite and metatorbernite. Ondruš and Hyršl (Reference Ondruš and Hyršl1989) described duftite, posnjakite, wulfenite and a mineral belonging to the mixite group, later determined as agardite-(Ce) by Sejkora et al. (Reference Sejkora, Toegel and Pauliš2008).
The specimen containing vrančiceite comprises earlier hematite-rich calcite separated distinctly from later milky-white calcite. The earlier strongly hematitised calcite hosts aggregates of hedyphane and Cu-sulfides up to 1 mm in size. The band of later milky-white calcite up to 2.5 cm thick, besides vrančiceite, contains up to 1 cm large fragments of the earlier hematitised calcite strongly intergrown with hedyphane, white or yellowish hedyphane aggregates up to 7 mm in size, cinnabar grains up to 2 mm across, djurleite aggregates up to 5 mm in length, and grains of galena up to 200 μm in size. The crystallisation of vrančiceite is related to a low-T (<100°C) hydrothermal event.
Physical and optical properties
Vrančiceite forms anhedral grains up to 100 μm in size (Fig. 1). The mineral is black in colour and opaque in transmitted light; it has a metallic lustre. A distinct cleavage in one direction was observed; it is brittle with a conchoidal fracture. The calculated density for the empirical formula (Z = 2) is 6.652 g.cm–3. Mohs hardness is assumed to be 2–3, similar to the associated cinnabar and djurleite. In reflected light, vrančiceite is light grey with a yellowish shade; bireflectance, pleochroism and anisotropy are all weak. Internal reflections were not observed. Reflectance spectra were measured in air with a TIDAS MSP400 spectrophotometer attached to a Leica microscope (100× objective) using a WTiC (Zeiss no. 370) standard, with a square sample measurement field of ca. 4 × 4 μm. The results for the 400–700 nm range are given in Table 1 and plotted in Fig. 2.

Figure 1. Back-scattered electron image of the holotype material (catalogue number P1P 42/2022). Vrančiceite is medium grey, whereas djurleite is dark grey and light grey are cinnabar and galena. The red box indicates the area where the grain used for single-crystal X-ray diffraction was extracted.
Table 1. Reflectance values (%) for vrančiceite.*

* The reference wavelengths required by the Commission on Ore Mineralogy (COM) are given in bold.
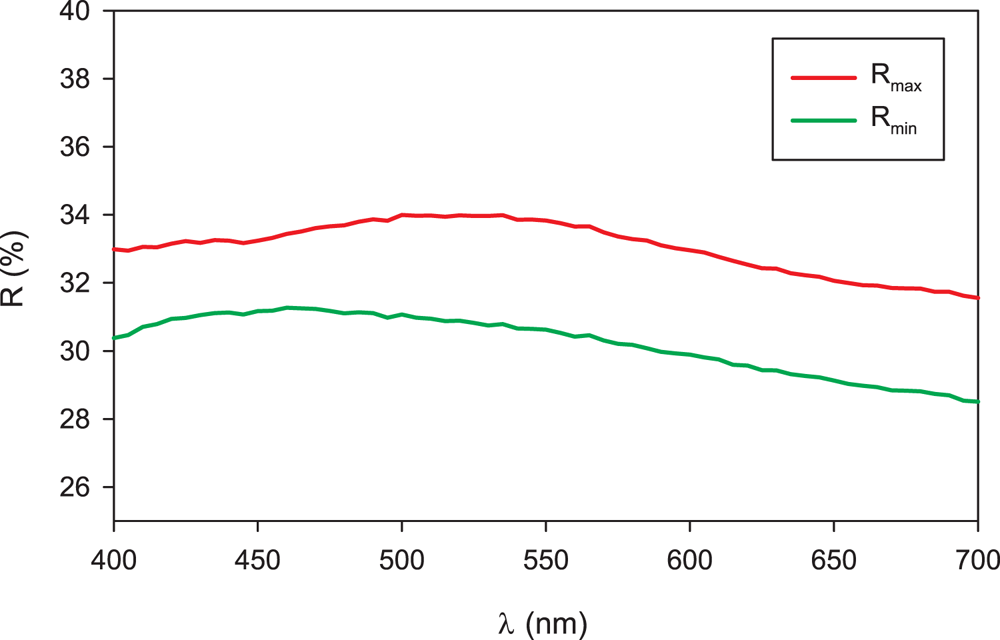
Figure 2. Reflectance curve for vrančiceite.
Chemical composition
Chemical analyses were performed using a Cameca SX100 electron microprobe operating in wavelength-dispersive mode (20 kV, 20 nA and 1 μm beam size). The following standards and X-ray lines were used to minimise line overlaps: Ag (AgLα), Bi (BiLα), Bi2Se3 (SeLβ), Cd (CdLα), chalcopyrite (CuKα, FeKα and SKα), FeAsS (AsKβ), HgS (HgLα), NaCl (ClKα), PbS (PbMα), Sb2S3 (SbLβ), Sn (SnLβ) and ZnS (ZnKα). Peak counting times were 20 s for all elements, and 10 s for each background. Arsenic, Cd, Cl, Fe, Pb, Se, Sn and Zn were all found to be below the detection limits (0.02–0.05 wt.%). Raw intensities were converted to the concentrations of elements using the automatic ‘PAP’ (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) matrix-correction procedure.
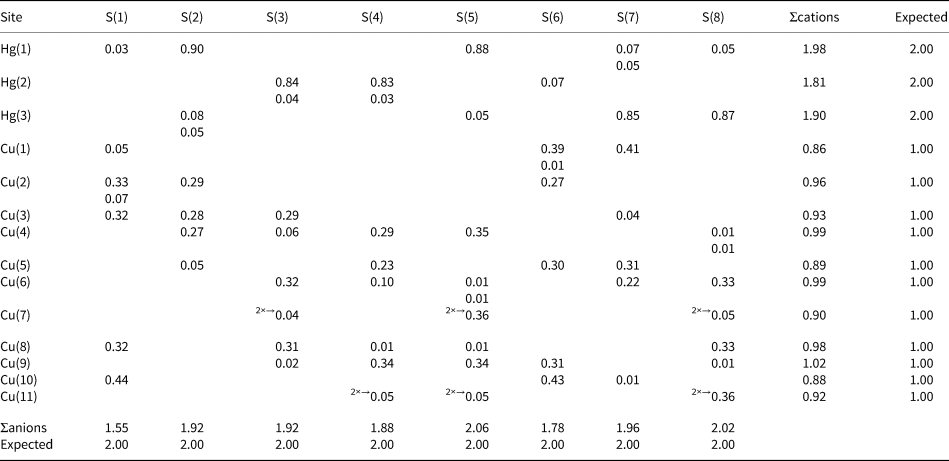
Analytical data for vrančiceite (6 analyses) are given in Table 2. On the basis of ΣMe = 13 atoms per formula unit the empirical chemical formula is Cu10.11(4)Ag0.01(1)Hg2.87(4)Sb0.01(1)Bi0.01(1)S7.99(8). The ideal formula is Cu10Hg3S8, which requires Cu 42.54, Hg 40.29 and S 17.17, a total of 100.00 wt.%.
Table 2. Electron-microprobe analyses and chemical data (wt.%) for vrančiceite (n = 6).
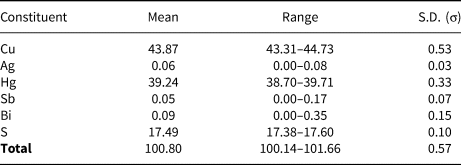
S.D. – standard deviation
X-ray diffraction data
A short prismatic fragment of vrančiceite, 60 × 40 × 30 μm in size, extracted from the polished section analysed using an electron microprobe (Fig. 1), was mounted on a glass fibre and examined with a Bruker D8 Venture single-crystal diffractometer equipped with an air-cooled Photon III area detector and microfocus MoKα radiation (Centro per l'Integrazione della Strumentazione Scientifica dell'Università di Pisa, University of Pisa). The detector-to-crystal distance was 38 mm. Data were collected using ω and φ scan modes, in 0.5° slices, with an exposure time of 15 s per frame. A total of 3462 frames were collected and they were integrated with the Bruker SAINT software package using a narrow-frame algorithm. Data were corrected for Lorentz-polarisation, absorption and background. Unit-cell parameters, refined on the basis of the XYZ centroids of 9884 reflections above 20σ(I) with 5.30 < 2θ < 62.06°, are a = 7.9681(2), b = 9.7452(3), c = 10.0710(3) Å, α = 77.759(1), β = 76.990(1), γ = 79.422(1)° and V = 737.01(4) Å3. The a:b:c ratio calculated from unit-cell parameters is 0.8176:1:1.0335. The statistical test on the distribution of the |E| values (|E 2 – 1| = 0.931) is in accord with the centric nature of vrančiceite. The crystal structure of vrančiceite was then solved in the space group P $\bar{1}$![]() using ShelxTL and refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015). Three independent Hg sites, eleven Cu positions, and eight S sites were located. Neutral-scattering curves for Hg, Cu and S sites were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). After several cycles of isotropic refinement, the R 1 factor converged to 0.0993, suggesting the correctness of the structural model. All sites were found fully occupied and their site occupancies were then fixed to 1. At the last stage, the anisotropic structural model converged to R = 0.0262 for 4212 reflections with F o > 4σ(F o) and 193 refined parameters. Details of data collection and refinement are given in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4. Table 5 reports selected bond distances, whereas bond-valence sums (BVS), calculated according to Brese and O'Keeffe (Reference Brese and O'Keeffe1991), are shown in Table 6. Anisotropic displacement parameters are reported in the Crystallographic Information File, deposited with the Principal Editor of Mineralogical Magazine and available as Supplementary material (see below).
using ShelxTL and refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015). Three independent Hg sites, eleven Cu positions, and eight S sites were located. Neutral-scattering curves for Hg, Cu and S sites were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). After several cycles of isotropic refinement, the R 1 factor converged to 0.0993, suggesting the correctness of the structural model. All sites were found fully occupied and their site occupancies were then fixed to 1. At the last stage, the anisotropic structural model converged to R = 0.0262 for 4212 reflections with F o > 4σ(F o) and 193 refined parameters. Details of data collection and refinement are given in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4. Table 5 reports selected bond distances, whereas bond-valence sums (BVS), calculated according to Brese and O'Keeffe (Reference Brese and O'Keeffe1991), are shown in Table 6. Anisotropic displacement parameters are reported in the Crystallographic Information File, deposited with the Principal Editor of Mineralogical Magazine and available as Supplementary material (see below).
Table 3. Summary of data collection conditions and refinement parameters for vrančiceite.
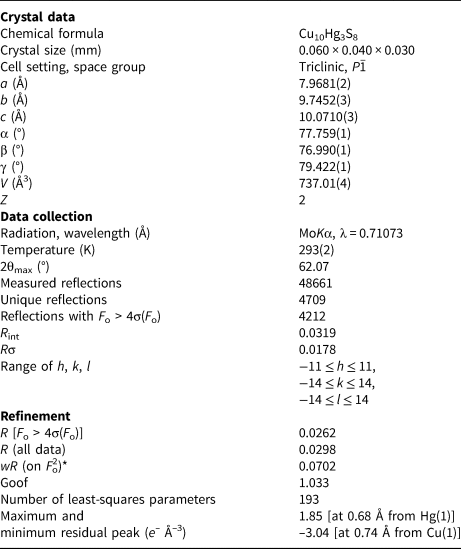
*w = 1/[σ2(F o2)+(0.0318P)2+8.6260P], where P = (F o2+2F c2)/3
Table 4. Sites, Wyckoff position (Wyc.), fractional atom coordinates, equivalent isotropic displacement parameters (Å2) for vrančiceite.
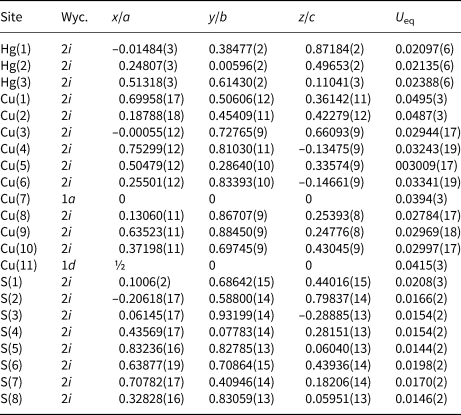
Table 5. Selected Cu–S and Hg–S distances (in Å) for vrančiceite.
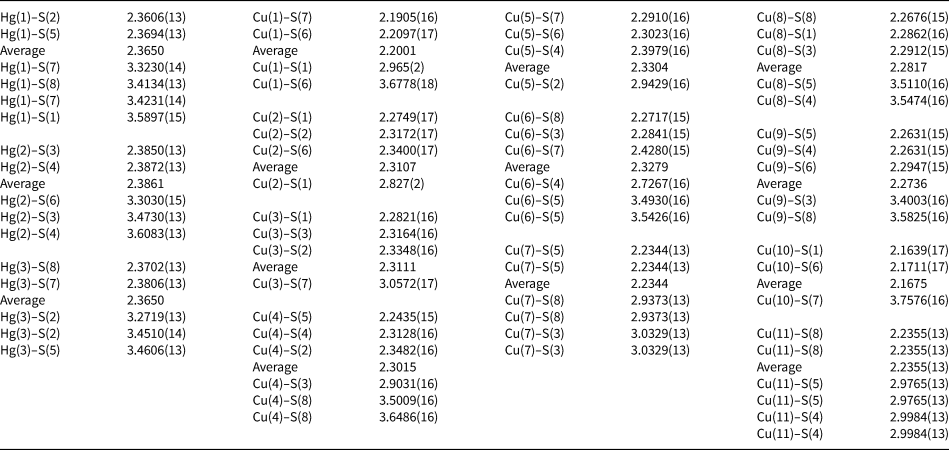
Table 6. Bond valence (in valence units) in vrančiceite.
Powder X-ray diffraction data could not be collected, due to the paucity of available material. Consequently, powder X-ray diffraction data, given in Table 7, were calculated using the software PowderCell 2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural model given in Tables 3 and 4.
Table 7. Calculated powder X-ray diffraction data for vrančiceite.*

* Intensity and d hkl (in Å) were calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural data given in Tables 3 and 4. Only reflections with I rel. ≥ 10 are listed. The six strongest reflections are given in bold.
Crystal structure of vrančiceite
Vrančiceite displays a new crystal structure type among Cu–Hg chalcogenides. It can be described as {0$\bar{1}$![]() 1} Cu–S layers, connected through CuS3 polyhedra, giving rise to a three-dimensional framework with channels running along the a axis and hosting linearly coordinated Hg atoms (Fig. 3). In addition to Cu–S and Hg–S bonds, there are some short Cu–Cu contacts, probably playing a role in the coordination environments of Cu atoms, as observed in other Cu phases, e.g. in gortdrumite (Bindi et al., Reference Bindi, Paar and Leblhuber2018).
1} Cu–S layers, connected through CuS3 polyhedra, giving rise to a three-dimensional framework with channels running along the a axis and hosting linearly coordinated Hg atoms (Fig. 3). In addition to Cu–S and Hg–S bonds, there are some short Cu–Cu contacts, probably playing a role in the coordination environments of Cu atoms, as observed in other Cu phases, e.g. in gortdrumite (Bindi et al., Reference Bindi, Paar and Leblhuber2018).

Figure 3. Crystal structure of vrančiceite as seen down the a axis. Blue, violet and yellow circles are Cu, Hg and S atoms, respectively. Hg–S bonds are shown as thick red lines, whereas thin black lines indicate Cu–S bonds. The unit cell is shown with dashed lines. Drawn using CrystalMaker® software.
In the crystal structure of vrančiceite there are 14 cation sites. Among these, the Cu atoms are hosted at 11 symmetry-independent positions, showing variable coordinations. Considering the shortest (= the strongest) Cu–S bonds, Cu coordinations can be described as quasi-linear or triangular. Four Cu atoms, Cu(1), Cu(7), Cu(10) and Cu(11) sites, have quasi-linear coordination. Their average bond distances range between 2.17 Å and 2.24 Å, whereas S–Cu–S angles vary between 144.77(8)° and 180°. These bond distances can be compared with those observed in linearly-coordinated Cu atoms in balkanite (2.197 Å – Biagioni and Bindi, Reference Biagioni and Bindi2017) as well as in other Cu sulfides, e.g. djurleite (2.19 Å – Evans, Reference Evans1979), and pearceite–polybasite-series minerals (2.16–2.17 Å; Bindi et al., Reference Bindi, Evain and Menchetti2006; Evain et al., Reference Evain, Bindi and Menchetti2006). Bond-valence sums at these Cu sites range between 0.72 and 0.87 valence units (vu). Taking into account longer Cu–S contacts, the BVS at Cu sites increases, varying between 0.86 and 0.92 vu. Seven triangularly coordinated Cu sites were found in the crystal structure of vrančiceite: Cu(2), Cu(3), Cu(4), Cu(5), Cu(6), Cu(8) and Cu(9). Average bond distances are in the range 2.27–2.33 Å, with bond-valence sums between 0.84 and 0.99 vu. These average bond distances for triangularly coordinated Cu atoms can be compared with those observed in other Cu sulfides, e.g. in some tetrahedrite-group minerals (2.259 Å – Wuensch, Reference Wuensch1964; 2.262 Å – Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020; 2.251 Å – Biagioni et al., Reference Biagioni, Sejkora, Musetti, Makovicky, Pagano, Pasero and Dolníček2022). In gortdrumite, Bindi et al. (Reference Bindi, Paar and Leblhuber2018) reported longer Cu–S bond distances (2.38 Å), whereas three-fold coordinated Cu atoms in balkanite have average distances similar to those observed in vrančiceite, varying between 2.26 and 2.32 Å (Biagioni and Bindi, Reference Biagioni and Bindi2017).
Several short Cu–Cu contacts are also present, for both two- and three-fold coordinated Cu atoms. All Cu sites, with the exception of Cu(8), Cu(9) and Cu(11), display one or two Cu–Cu distances shorter than 2.75 Å, with some short values such as that shown by the pair Cu(1)–Cu(10), i.e. 2.6012(15) Å, slightly longer than that observed in metallic copper, i.e. 2.55 Å (Suh et al., Reference Suh, Ohta and Waseda1988). These Cu–Cu contacts are longer than those observed in balkanite (Biagioni and Bindi, Reference Biagioni and Bindi2017) and similar to some Cu–Cu distances reported by Bindi et al. (Reference Bindi, Paar and Leblhuber2018) in gortdrumite. Also the Cu–Hg selenide brodtkorbite shows short Cu–Cu contacts, ranging between 2.535 and 2.670 Å (Sejkora et al., Reference Sejkora, Škácha, Laufek and Plášil2017).
The three independent Hg sites have linear coordination, with average <Hg–S> distances ranging between 2.36 and 2.39 Å, and S–Hg–S angles varying between 173.07(5)° and 179.49(4)°. These distances are comparable with those observed in other minerals characterised by linearly-coordinated Hg, e.g. cinnabar (2.368 Å – Auvray and Genet, Reference Auvray and Genet1973), imiterite (2.376 Å – Guillou et al., Reference Guillou, Monthel, Picot, Pillard, Protas and Samama1985), rouxelite (2.381 Å – Orlandi et al., Reference Orlandi, Meerschaut, Moëlo, Palvadeau and Léone2005), marrucciite (2.361 and 2.386 Å for the two independent Hg1 and Hg2 sites – Orlandi et al., Reference Orlandi, Moëlo, Campostrini and Meerschaut2007), fettelite (2.403 and 2.393 Å for the two independent Hg1 and Hg2 sites – Bindi et al., Reference Bindi, Keutsch, Francis and Menchetti2009), balkanite (2.366 Å – Biagioni and Bindi, Reference Biagioni and Bindi2017), and gortdrumite (2.386 Å, as average of five Hg sites – Bindi et al., Reference Bindi, Paar and Leblhuber2018). Longer Hg–S bonds (> 3.25 Å) complete the coordination environments of Hg atoms. Taking into account Hg–S distances shorter than 3.65 Å, Hg(1) has a 2 + 4 coordination, whereas Hg(2) and Hg(3) display a 2 + 3 coordination. Considering the long (= weak) bonds, the bond-valence sums at the Hg sites range between 1.81 and 1.98 vu. It is worth noting that these weak Hg–S bonds are longer than some Hg–Cu contacts, showing distances in the range 3.10–3.20 Å.
Anion sites are fully occupied by S and are four-fold coordinated, with additional longer Cu–S or Hg–S bonds. Bond-valence sums are in the range 1.55–2.06 vu.
The occurrence of several metal–metal contacts suggests that vrančiceite could be considered as a peculiar sulfide having some features typical of intermetallic compounds, as noted for gortdrumite by Bindi et al. (Reference Bindi, Paar and Leblhuber2018). Notwithstanding, the bond-valence considerations seem to be applicable and the results of the bond-valence balance are in reasonable agreement with the expected values (Table 6).
Discussion
Vrančiceite and related Cu–Hg sulfides
Vrančiceite is a new member of the Cu–Hg–S system. Ollitrault-Fichet et al. (Reference Ollitrault-Fichet, Rivet and Flahaut1984) examined the phase diagram of this system but found nothing similar to vrančiceite. They found a ternary phase with composition Cu0.42Hg0.18S0.39 that was only stable at high temperature. The absence of the synthetic analogue of vrančiceite in the experiments of Ollitrault-Fichet et al. (Reference Ollitrault-Fichet, Rivet and Flahaut1984) suggests that it could be formed at low temperatures.
Among natural Cu–Hg–S phases, ternary compounds are represented by two species: the inadequately described ‘bayankhanite’, from the fluorite deposit of Idermeg-Bayan-Khan-Ula, Mongolia (Kuznetsov et al., Reference Kuznetsov, Obolenskiy, Vasil'ev and Borisenko1978; Vasil'ev, Reference Vasil'ev1984), and danielsite, (Cu,Ag)14HgS8 (Nickel, Reference Nickel1987; Kato and Nickel, Reference Kato and Nickel1988). Three published chemical analyses of the former show a very wide range of Cu and Hg, ranging between 20.9 and 40.9 wt.% and 43 and 59 wt.%, respectively. Among these analyses, one has Cu 40.9, Hg 43.0 and S 17.0 wt.%, close to the composition of vrančiceite (Fig. 4); in addition, the powder X-ray diffraction data of ‘bayankhanite’ are of very low quality, although similar to those of vrančiceite (Vasil'ev, Reference Vasil'ev1984). On the other hand, its optical properties (strong bireflectance and anisotropy) are fundamentally different from vrančiceite. In any case, ‘bayankhanite’ was discredited by Burke (Reference Burke2006). Danielsite is poorly characterised, and its actual relationship with balkanite is unclear (Biagioni and Bindi, Reference Biagioni and Bindi2017); as shown in Fig. 4, their composition is very similar.
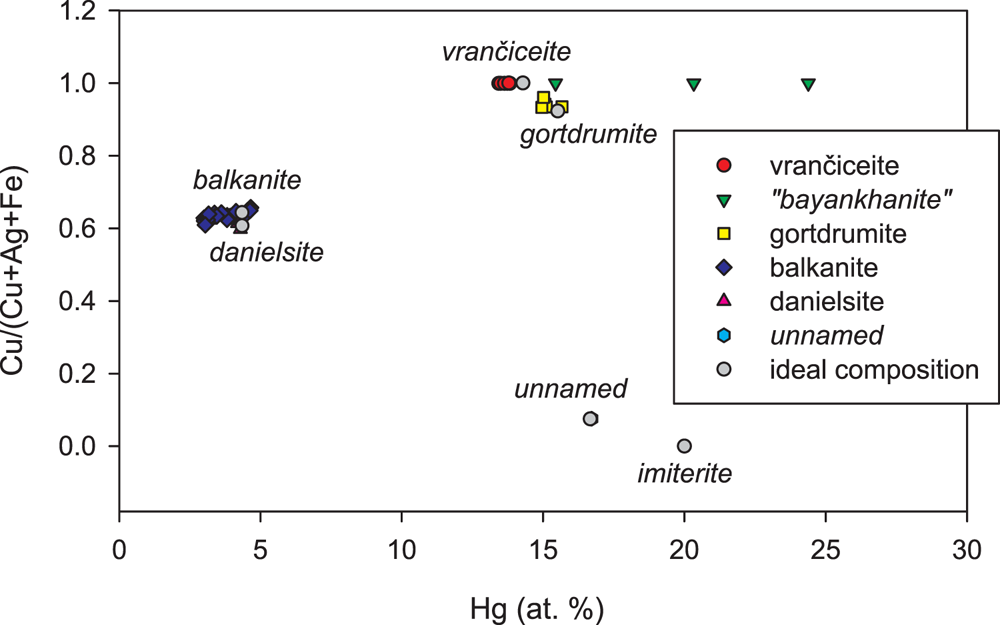
Figure 4. Chemical composition of minerals of the system Cu–Ag–Hg–S in the plot Hg (at.%) vs. Cu/(Cu+Ag+Fe) (at. units). Ideal compositions and published data: vrančiceite Cu10Hg3S8 (this paper); discredited ‘bayankhanite’ (Kuznetsov et al., Reference Kuznetsov, Obolenskiy, Vasil'ev and Borisenko1978; Vasil'ev, Reference Vasil'ev1984); gortdrumite Cu24Fe2Hg9S23 (Steed, Reference Steed1983; Bindi et al., Reference Bindi, Paar and Leblhuber2018); balkanite Cu9Ag5HgS8 (Atanassov and Kirov, Reference Atanassov and Kirov1973; Paar and Chen, Reference Paar and Chen1985; Steiner et al., Reference Steiner, Tropper, Vavtar, Kaindl and Krismer2010; Biagioni and Bindi, Reference Biagioni and Bindi2017; Sejkora et al., Reference Sejkora, Dolníček, Škácha, Ulmanová and Vrtiška2022); danielsite (Cu8.5Ag5.5)Σ14HgS8 (Nickel, Reference Nickel1987; Kato and Nickel, Reference Kato and Nickel1988), unnamed (Ag7.4Cu0.6)Σ8Hg3(S,Se)7 (Paar et al., Reference Paar, Topa, Makovicky, Sureda, De Brodtkorb, Nickel and Putz2004); and imiterite Ag2HgS2 (Guillou et al., Reference Guillou, Monthel, Picot, Pillard, Protas and Samama1985).
Vrančiceite has an Hg content (in atom%) and a Cu/(Cu+Ag+Fe) atomic ratio close to that of gortdrumite. Indeed, as discussed in the Introduction, Škácha and Sejkora (Reference Škácha and Sejkora2013) first identified vrančiceite as an Fe-free gortdrumite. Their structural relationship deserves further discussion.
Relations between vrančiceite and gortdrumite
The crystal structure of gortdrumite, reported by Bindi et al. (Reference Bindi, Paar and Leblhuber2018), can be described as a succession of {$\bar{1}$![]() 10} layers. As in vrančiceite, linearly coordinated Hg atoms are hosted in channels running along the c axis (Fig. 5). A comparison between the projections of the crystal structures of vrančiceite and gortdrumite (Figs 3 and 5) reveals a striking similarity in the layer morphology as well as in the directions of Hg–S bonds. Moreover, the two crystal structures can be better compared transforming the axial setting of gortdrumite through the matrix [0 0 1 | 1 0 0 | 0 1 0]. Table 8 compares the unit-cell parameters of these two species. Whereas the transformed b and c axes of gortdrumite are slightly shorter than that of vrančiceite, the a axis of the former is significantly longer than that of the latter, explaining the volume increase observed in gortdrumite (ΔV/V = +46.1%).
10} layers. As in vrančiceite, linearly coordinated Hg atoms are hosted in channels running along the c axis (Fig. 5). A comparison between the projections of the crystal structures of vrančiceite and gortdrumite (Figs 3 and 5) reveals a striking similarity in the layer morphology as well as in the directions of Hg–S bonds. Moreover, the two crystal structures can be better compared transforming the axial setting of gortdrumite through the matrix [0 0 1 | 1 0 0 | 0 1 0]. Table 8 compares the unit-cell parameters of these two species. Whereas the transformed b and c axes of gortdrumite are slightly shorter than that of vrančiceite, the a axis of the former is significantly longer than that of the latter, explaining the volume increase observed in gortdrumite (ΔV/V = +46.1%).

Figure 5. (a) Crystal structure of gortdrumite as seen down the c axis. Blue, violet and yellow circles are Cu, Hg and S atoms, respectively; Fe-centred tetrahedra are shown in brown. Hg–S bonds are shown as thick red lines, whereas thin black lines indicate Cu–S bonds. (b) The {0$\bar{1}$![]() 1} Cu–S layer in vrančiceite, as seen down a and perpendicular to the layer. (c) The {$\bar{1}$
1} Cu–S layer in vrančiceite, as seen down a and perpendicular to the layer. (c) The {$\bar{1}$![]() 10} Cu–Fe–S layer in gortdrumite, as seen down c and perpendicular to the layer. Drawn using CrystalMaker® software.
10} Cu–Fe–S layer in gortdrumite, as seen down c and perpendicular to the layer. Drawn using CrystalMaker® software.
Table 8. Comparison between unit-cell parameters in vrančiceite and gortdrumite.
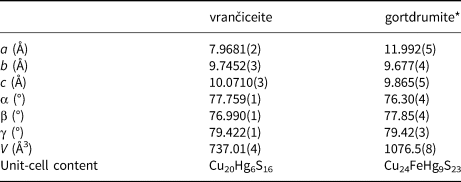
*unit-cell parameters transformed through the matrix [0 0 1 | 1 0 0 | 0 1 0].
The Cu–(Fe)–S layers of vrančiceite and gortdrumite are compared in Figs 5b and c. Along the channel directions (upper part of Figs 5b and c), the layers can be divided into two portions: the first one is a two-atom-thick zig-zag ribbon, whereas the second one has an elliptical shape. Figure 6 shows the differences between these layers in vrančiceite and gortdrumite. In both minerals, the zig-zag ribbons are six-atoms-wide (Fig. 6a,b); however, their chemical composition is different, with gortdrumite showing the insertion of a Cu4Fe2S6 fragment and the omission of 2 Cu atoms [corresponding to the Cu(1) atom in vrančiceite]. Consequently, the chemistry of the zig-zag ribbons in vrančiceite and gortdrumite is Cu14S12 and Cu16Fe2S18, respectively. The remaining portion of the layers has a composition of Cu6S4 in vrančiceite and Cu8S5 in gortdrumite (Fig. 6c,d). Also in this case, the intercalation of a Cu2S fragment increases the parameter along the ribbon elongation of gortdrumite. As a whole, the Cu–(Fe)–S layers have a chemical composition of Cu14S12 + Cu6S4 = Cu20S16 in vrančiceite and Cu16Fe2S18 + Cu8S5 = Cu24Fe2S23 in gortdrumite.

Figure 6. Details of the Cu–(Fe)–S layers in vrančiceite (a, c) and gortdrumite (b, d), showing the insertion of structural fragments in the latter (highlighted in blue). In (a) and (b), the zig-zag ribbons are shown perpendicularly to their layering, whereas in (c) and (d) the remaining portion of the layers are displayed. Drawn using CrystalMaker® software.
In both minerals, these layers are decorated, on both sides, by Hg atoms. The increased size of the gortdrumite layers favours the addition of further Hg atoms with respect to vrančiceite. Indeed, three additional Hg atoms occur in the former, having the unit-cell content Cu24Fe2Hg9S23, compared with the unit-cell content of vrančiceite, Cu20Hg6S16.
Conclusions
Vrančiceite is a new ternary phase in the Cu–Hg–S system. Its discovery and comparison with previously known Cu–Hg chalcogenides confirm the fundamental role of studies devoted to natural mineral assemblages to reveal novel crystal structures so far not obtained in laboratory synthesis experiments (e.g. Ollitrault-Fichet et al., Reference Ollitrault-Fichet, Rivet and Flahaut1984; Bindi et al., Reference Bindi, Nespolo, Krivovichev, Chapuis and Biagioni2020).
Vrančiceite is a contracted derivative of gortdrumite, obtained through the omission of a zig-zag Fe-bearing structural fragment. These two minerals can thus be considered a new example of a plesiotypic pair (e.g. Makovicky, Reference Makovicky1997; Ferraris et al., Reference Ferraris, Makovicky and Merlino2004). Moreover, this is an additional case showing the role of minor elements in the crystallisation of different mineral species: the occurrence or absence of minor Fe can favour the formation of gortdrumite [Fe/(Fe+Cu+Hg)at. ratio = 0.06] or vrančiceite [Fe/(Fe+Cu+Hg)at. ratio = 0.00], that consequently reflects the variable geochemistry of hydrothermal environments.
Acknowledgements
The helpful comments of two anonymous reviewers, Peter Leverett, Associate Editor Ian Terence Graham and Principal Editor Stuart Mills are greatly appreciated. The study was financially supported by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2019-2023/1.II.e; National Museum, 00023272) and the Czech Science Foundation (project 19-16218S) for JS and PŠ, and by the Ministero dell'Istruzione, Università e Ricerca (project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32) for CB.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2023.40.
Competing interests
The authors declare none.