Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Siidra, Oleg I.
Vergasova, Lidiya P.
Kretser, Yuri L.
Polekhovsky, Yuri S.
Filatov, Stanislav K.
and
Krivovichev, Sergey V.
2014.
Unique thallium mineralization in the fumaroles of Tolbachik volcano, Kamchatka Peninsula, Russia. III. Evdokimovite, Tl4(VO)3(SO4)5(H2O)5.
Mineralogical Magazine,
Vol. 78,
Issue. 7,
p.
1711.
Siidra, Oleg I.
Vergasova, Lidiya P.
Kretser, Yuri L.
Polekhovsky, Yuri S.
Filatov, Stanislav K.
and
Krivovichev, Sergey V.
2014.
Unique thallium mineralization in the fumaroles of the Tolbachik volcano, Kamchatka Peninsula, Russia. II. Karpovite, Tl2VO(SO4)2(H2O).
Mineralogical Magazine,
Vol. 78,
Issue. 7,
p.
1699.
Bindi, Luca
Biagioni, Cristian
Martini, Bruno
Salvetti, Adrio
Fontana, Giovanni Dalla
Taronna, Massimo
and
Ciriotti, Marco E.
2016.
Tavagnascoite, Bi4O4(SO4)(OH)2, a new oxyhydroxy bismuth sulfate related to klebelsbergite.
Mineralogical Magazine,
Vol. 80,
Issue. 4,
p.
647.
Kovrugin, Vadim M.
Siidra, Oleg I.
Pekov, Igor V.
Chukanov, Nikita V.
Khanin, Dmitry A.
and
Agakhanov, Atali A.
2018.
Embreyite: structure determination, chemical formula and comparative crystal chemistry.
Mineralogical Magazine,
Vol. 82,
Issue. 2,
p.
275.
Pekov, I.V.
Agakhanov, A.A.
Zubkova, N.V.
Koshlyakova, N.N.
Shchipalkina, N.V.
Sandalov, F.D.
Yapaskurt, V.O.
Turchkova, A.G.
and
Sidorov, E.G.
2020.
Oxidizing-Type Fumaroles of the Tolbachik Volcano, a Mineralogical and Geochemical Unique.
Russian Geology and Geophysics,
Vol. 61,
Issue. 5-6,
p.
675.
Inostroza, Manuel
Aguilera, Felipe
Menzies, Andrew
Layana, Susana
González, Cristóbal
Ureta, Gabriel
Sepúlveda, José
Scheller, Samuel
Böehm, Stephan
Barraza, María
Tagle, Roald
and
Patzschke, Max
2020.
Deposition of metals and metalloids in the fumarolic fields of Guallatiri and Lastarria volcanoes, northern Chile.
Journal of Volcanology and Geothermal Research,
Vol. 393,
Issue. ,
p.
106803.
Siidra, Oleg I.
Nazarchuk, Evgeny V.
Zaitsev, Anatoly N.
and
Shilovskikh, Vladimir V.
2020.
Majzlanite, K2Na(ZnNa)Ca(SO4)4, a new anhydrous sulfate mineral with complex cation substitutions from Tolbachik volcano.
Mineralogical Magazine,
Vol. 84,
Issue. 1,
p.
153.
Gołębiowska, Bożena
Pieczka, Adam
Zubko, Maciej
Voegelin, Andreas
Göttlicher, Jörg
and
Rzepa, Grzegorz
2021.
Thalliomelane, TIMn7.54+Cu0.52+O16, a new member of the coronadite group from the preglacial oxidation zone at Zalas, southern Poland.
American Mineralogist,
Vol. 106,
Issue. 12,
p.
2020.
Siidra, Oleg I.
Borisov, Artem S.
Charkin, Dmitri O.
Depmeier, Wulf
and
Platonova, Natalia V.
2021.
Evolution of fumarolic anhydrous copper sulfate minerals during successive hydration/dehydration.
Mineralogical Magazine,
Vol. 85,
Issue. 2,
p.
262.
Zhao, Fengqi
and
Gu, Shangyi
2021.
Secondary Sulfate Minerals from Thallium Mineralized Areas: Their Formation and Environmental Significance.
Minerals,
Vol. 11,
Issue. 8,
p.
855.
Wei, Feixiang
Prytulak, Julie
Baker, Evelyn B.
Xu, Jiandong
and
Zhao, Bo
2022.
Identifying Tethys oceanic fingerprint in post-collisional potassium-rich lavas in Tibet using thallium isotopes.
Chemical Geology,
Vol. 607,
Issue. ,
p.
121013.
Vereshchagin, Oleg S.
Frank-Kamenetskaya, Olga V.
Yu. Vlasov, Dmitry
Zelenskaya, Marina S.
Rodina, Oksana A.
Chernyshova, Irina A.
Himelbrant, Dmitry E.
Stepanchikova, Irina S.
and
Britvin, Sergey N.
2023.
Microbial biomineralization under extreme conditions: Case study of basaltic rocks, Tolbachik Volcano, Kamchatka, Russia.
CATENA,
Vol. 226,
Issue. ,
p.
107048.
Pekov, Igor V.
Krzhizhanovskaya, Maria G.
Yapaskurt, Vasiliy O.
Belakovskiy, Dmitry I.
Sidorov, Evgeny G.
and
Zhegunov, Pavel S.
2023.
Kalithallite, K3Tl3+Cl6⋅2H2O, a new mineral with trivalent thallium from the Tolbachik volcano, Kamchatka, Russia.
Mineralogical Magazine,
Vol. 87,
Issue. 2,
p.
186.
Inostroza, Manuel
Moune, Séverine
Aguilera, Felipe
Vlastelic, Ivan
Burckel, Pierre
Tapia, Joseline
Irarrázabal, Nahun
and
Fernández, Bárbara
2024.
Lastarria volcano, a major emitter of boron and chalcophiles in northern Chile and the Central Volcanic Zone.
Chemical Geology,
Vol. 670,
Issue. ,
p.
122416.
Chen, Pengyun
2024.
On the Structure of Tl2Pb(SO4)2 and Crystal Chemistry of Thallium‐Containing Sulfates.
Zeitschrift für anorganische und allgemeine Chemie,
Vol. 650,
Issue. 19,
Borisov, Artem S.
Siidra, Oleg I.
Vlasenko, Natalia S.
Platonova, Natalia V.
Schuldt, Thies
Neuman, Mason
Strauss, Harald
and
Holzheid, Astrid
2024.
The Yadovitaya fumarole, Tolbachik volcano: A comprehensive mineralogical and geochemical study and driving factors for mineral diversity.
Geochemistry,
Vol. 84,
Issue. 3,
p.
126179.
Prytulak, Julie
and
König, Stephan
2025.
Treatise on Geochemistry.
p.
671.
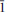 , a = 7.378(3), b = 10.657(3), c = 10.657(3) Å , α = 61.31(3), β = 70.964(7), γ = 70.964(7)º, V = 680.2(4) Å3, Z = 4 (from single-crystal diffraction data). The eight strongest lines of the X-ray powder diffraction pattern are (I/d/hkl): 68/4.264/111, 100/3.441/113, 35/3.350/222, 24/3.125/122, 23/3.054/202, 45/2.717/022, 20/2.217/331, 34/2.114/204. Chemical composition determined by electron microprobe analysis is (wt.%): Tl2O 35.41, Bi2O3 38.91, SO3 25.19, total 99.51. The empirical formula based on 8 O a.p.f.u. is Tl1.04Bi1.05S1.97O8. The simplified formula is TlBi(SO4)2, which requires Tl2O 35.08, Bi2O3 38.48, SO3 26.44, total 100.00 wt.%. The crystal structure was solved by direct methods and refined to R1 = 0.055 on the basis of 1425 independent observed reflections. The structure contains four Tl+ and two Bi3+ sites in holodirected symmetrical coordination. BiO8 tetragonal antiprisms and SO4 tetrahedra in markhininite share common O atoms to produce [Bi(SO4)2]– layers of the yavapaiite type. The layers are parallel to (111) and linked together through interlayer Tl+ cations. The mineral is named in honour of Professor Yevgeniy Konstantinovich Markhinin (b. 1926), Institute of Volcanology, Russian Academy of Sciences, Kamchatka peninsula, Russia, in recognition of his contributions to volcanology. Markhininite is the first oxysalt compound that contains both Tl and Bi in an ordered crystal structure.
, a = 7.378(3), b = 10.657(3), c = 10.657(3) Å , α = 61.31(3), β = 70.964(7), γ = 70.964(7)º, V = 680.2(4) Å3, Z = 4 (from single-crystal diffraction data). The eight strongest lines of the X-ray powder diffraction pattern are (I/d/hkl): 68/4.264/111, 100/3.441/113, 35/3.350/222, 24/3.125/122, 23/3.054/202, 45/2.717/022, 20/2.217/331, 34/2.114/204. Chemical composition determined by electron microprobe analysis is (wt.%): Tl2O 35.41, Bi2O3 38.91, SO3 25.19, total 99.51. The empirical formula based on 8 O a.p.f.u. is Tl1.04Bi1.05S1.97O8. The simplified formula is TlBi(SO4)2, which requires Tl2O 35.08, Bi2O3 38.48, SO3 26.44, total 100.00 wt.%. The crystal structure was solved by direct methods and refined to R1 = 0.055 on the basis of 1425 independent observed reflections. The structure contains four Tl+ and two Bi3+ sites in holodirected symmetrical coordination. BiO8 tetragonal antiprisms and SO4 tetrahedra in markhininite share common O atoms to produce [Bi(SO4)2]– layers of the yavapaiite type. The layers are parallel to (111) and linked together through interlayer Tl+ cations. The mineral is named in honour of Professor Yevgeniy Konstantinovich Markhinin (b. 1926), Institute of Volcanology, Russian Academy of Sciences, Kamchatka peninsula, Russia, in recognition of his contributions to volcanology. Markhininite is the first oxysalt compound that contains both Tl and Bi in an ordered crystal structure.