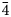Introduction
Tetrahedrite-group minerals are characterised by the general structural formula M (2)A6M (1)(B4C2)X (3)D4S(1)Y12S(2)Z, where the capital letters indicate several chemical constituents. Among the different species, the most common belong to the tetrahedrite and tennantite series and are characterised by A and B = Cu+, D = Sb3+ or As3+, and Y and Z = S2–. Different C constituents, usually represented by divalent transition elements, identify the species belonging to these series (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a).
The name ‘tetrahedrite’ was introduced by Haidinger (Reference Haidinger1845) in agreement with the common tetrahedral form of its crystals. Previously, tetrahedrite was known with different names, for instance fahlerz, fahlerts, weissgiltigerz, grey ore, or panabase. Haidinger (Reference Haidinger1845) reported the occurrence of Fe and Zn in tetrahedrite. Indeed, these two constituents are its most common divalent cations (e.g. Johnson et al., Reference Johnson, Craig and Rimstidt1986; George et al., Reference George, Cook and Ciobanu2017).
Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a) renamed these species as tetrahedrite-(Fe) and tetrahedrite-(Zn). Moreover, since the publication of the nomenclature of tetrahedrite-group minerals (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a), four other species belonging to the tetrahedrite series have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA-CNMNC), i.e. tetrahedrite-(Hg) (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b), tetrahedrite-(Mn) (Momma et al., Reference Momma, Shimizu, Kusaba and Ohki2022), tetrahedrite-(Ni) (Wang et al., Reference Wang, Chen, Gu, Nestola, Hou, Yang, Dong, Guo and Qu2023) and tetrahedrite-(Cd) (Sejkora et al., Reference Sejkora, Biagioni, Škácha, Musetti, Kasatkin and Nestola2023). Other potential end-member compositions are known in literature. Among them, Cu-dominant compositions corresponding to ideal Cu12Sb4S13 have been reported, for instance from Greece, France and the Czech Republic (Cesbron et al., Reference Cesbron, Giraud, Picot and Pillard1985; Repstock et al., Reference Repstock, Voudouris and Kolitsch2015; Voudouris et al., Reference Voudouris, Repstock, Spry, Frenzel, Mavrogonatos, Keith, Tarantola, Melfos, Tombros, Zhai, Cook, Ciobanu, Schaarschmidt, Rieck, Kolitsch and Falkenberg2022; Sejkora et al., Reference Sejkora, Biagioni, Škácha, Musetti, Kasatkin and Nestola2023). ‘Unsubstituted’ tetrahedrite–tennantite (i.e. without metals other than Cu and Ag) is also known from synthetic samples (Makovicky et al., Reference Makovicky, Karanović, Poleti, Balić-Žunić and Paar2005); the apparent excess of negative charges could be compensated by the presence of formally divalent Cu (Pattrick et al., Reference Pattrick, van der Laan, Vaughan and Henderson1993). According to Makovicky and Skinner (Reference Makovicky and Skinner1979), synthetic tetrahedrite Cu12+xSb4S13 (x varies continuously between < 0.1 and 1.9) exsolves, below 120°C, to a composition close to Cu12Sb4S13 (a = 10.32 Å) and to a Cu-excess composition, close to Cu14-xSb4S13 (x approximately equal to 0.2; a = 10.45 Å). The Cu-excess variety could be more common than previously thought, but, as stressed by Lind and Makovicky (Reference Lind and Makovicky1982), during electron-microprobe analysis a ‘loss’ of Cu over 12 atoms per formula unit (apfu) was observed, both in synthetic as well as natural samples of tetrahedrite and tennantite.
A new study of samples from the Slovak magnesite deposit Bankov near Košice (Peterec et al., Reference Peterec, Pauco and Horský1990) resulted in the description of the new mineral species tetrahedrite-(Cu). The new mineral and its name have been approved by the IMA–CNMNC, under the voting number IMA2022-078 (Sejkora et al., Reference Sejkora, Biagioni, Števko, Musetti and Peterec2022). Tetrahedrite-(Cu) is named after its chemical composition, in agreement with the nomenclature of the tetrahedrite group (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a). Its mineral symbol, in accord with Warr (Reference Warr2021), is Ttr-Cu. Holotype material of tetrahedrite-(Cu) is deposited in the collections of the Department of Mineralogy and Petrology, National Museum in Prague, Cirkusová 1740, 193 00 Praha 9, Czech Republic under the catalogue number P1P 27/2022 and in the collections of the Museo di Storia Naturale of the Università di Pisa, Via Roma 79, Calci (PI), under catalogue number 20017.
This work reports a description of this new mineral species, its position in the tetrahedrite group, and some crystal-chemical and nomenclature issues are discussed.
Occurrence and physical properties
Occurrence
Tetrahedrite-(Cu) was found at the stope K 401, in the 4th horizon of the Medvedza magnesite body, Bankov magnesite deposit near Košice (GPS coordinates: 48°44'8.06″N, 21°13'40.10″E), Košice Co., Košice Region, Slovak Republic. Hydrothermal-metasomatic bodies of magnesite at the Bankov deposit are hosted in Carboniferous shales and phyllites belonging to the Gemeric tectonic unit (Grecula et al., Reference Grecula, Abonyi, Abonyiová, Antaš, Bartalský, Bartalský, Dianiška, Ďuďa, Gargulák, Gazdačko, Hudáček, Kobulský, Lörincz, Macko, Návesňák, Németh, Novotný, Radvanec, Rojkovič, Rozložník, Varček and Zlocha1995). Here an interesting hydrothermal ore mineralisation, represented mostly by Cu sulfosalts (skinnerite, chalcostibite and various minerals of the tetrahedrite group), is developed in a small scale on younger fractures in the Medvedza magnesite body in the form of crystalline crusts and fillings (Peterec et al., Reference Peterec, Pauco and Horský1990). Tetrahedrite-(Cu) is associated with skinnerite, chalcostibite, famatinite, tetrahedrite-(Fe), zoned aggregates of tennantite-(Cu) to tennantite-(Fe) and minor chalcopyrite and marcasite. This unusual association of Cu sulfosalts has no other equivalent in the whole Western Carpathians and it is related to hydrothermal, most probably Alpine solutions strongly enriched in Cu, Sb and S and later stages of crystallisation also rich in As as well as minor amounts of Ge. The primary ore mineralisation is locally replaced by younger supergene minerals including chalcocite, native copper, malachite and azurite.
Physical and optical properties
Tetrahedrite-(Cu) forms anhedral grains up to 200 × 400 μm (Fig. 1). It is steel-grey in colour, with a black streak and metallic lustre. Mohs hardness was not measured, owing to the small size of the studied grain and the intimate association of other sulfides, but it should be close to 3½–4, in agreement with other members of the tetrahedrite group. Tetrahedrite-(Cu) is brittle, with a conchoidal fracture and an indistinct cleavage. Due to the small size of the studied grains and their admixure with other sulfides, density was not measured; on the basis of the empirical formula and the single crystal X-ray diffraction data, the calculated density is 5.029 g⋅cm–3.

Figure 1. Back-scattered electron images of tetrahedrite-(Cu), associated with chalcostibite (white) and tennantite-(Fe) (dark grey) (a). Inset (b) shows details of tetrahedrite-(Cu): red points correspond to tetrahedrite-(Cu), observed zonality reflects Cu–Zn–Fe and Sb–As substitutions; the rest of the grey aggregate (marked by blue points) is Fe-richer tetrahedrite-(Cu) with contents 0.89–0.99 apfu Fe and without Zn and As. The grain used for single-crystal X-ray diffraction study was extracted from the area of the red box. Holotype sample, catalogue number P1P 27/2022.
In reflected light, tetrahedrite-(Cu) is isotropic and grey, with a bluish shade (Fig. 2). Internal reflections were not observed. Reflectance values measured in air on the holotype sample using a spectrophotometer MSP400 Tidas with Leica microscope, with a 20× objective, are given in Table 1 and shown in Fig. 3, where the reflectance curve for tetrahedrite-(Cu) is compared with published data for related tetrahedrite-group minerals.

Figure 2. Reflected-light photo of grey tetrahedrite-(Cu) associated with chalcostibite (white) and tennantite-(Fe) (pink-brownish grey). Holotype sample, catalogue number P1P 27/2022.
Table 1. Reflectance values (%) for tetrahedrite-(Cu).*

* The reference wavelengths required by the Commission on Ore Mineralogy (COM) are given in bold.

Figure 3. Reflectance curves for tetrahedrite-(Cu) from the Bankov deposit, compared with published data for other tetrahedrite-series minerals: tetrahedrite-(Cu) (this paper); tetrahedrite-(Zn), Fresney d́Oisans, Isère, France (Criddle and Stanley, Reference Criddle and Stanley1993); tetrahedrite-(Fe), Frigido mine, Massa, Tuscany, Italy (Criddle and Stanley, Reference Criddle and Stanley1993); tetrahedrite-(Hg), Buca della Vena mine, Apuan Alps, Tuscany, Italy (Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2020b), tetrahedrite-(Ni), Luobusa, Tibet, China (Wang et al., Reference Wang, Chen, Gu, Nestola, Hou, Yang, Dong, Guo and Qu2023); tetrahedrite-(Cd), Radětice near Příbram, Czech Republic (Sejkora et al., Reference Sejkora, Biagioni, Škácha, Musetti, Kasatkin and Nestola2023).
Chemical composition
Quantitative chemical analyses were carried out using a Cameca SX 100 electron microprobe (National Museum of Prague, Czech Republic) and the following experimental conditions: wavelength-dispersive spectroscopy mode, accelerating voltage = 25 kV, beam current = 20 nA, beam diameter = 1 μm. Standards (element, emission line) were: chalcopyrite (CuKα, SKα), pyrite (FeKα), ZnS (ZnKα), NiAs (AsLβ) and Sb2S3 (SbLα). The contents of other sought elements with Z > 8 (Ag, Au, Bi, Cd, Co, Ga, Ge, Hg, In, Mn, Cl, Ni, Pb, Se, Sn, Te and Tl) were below detection limits. Matrix correction by the PAP procedure (Pouchou and Pichoir, Reference Pouchou and Pichoir1985) was applied to the data. Electron back-scattered images showed that tetrahedrite-(Cu) is slightly zoned due to Cu–Fe–Zn and As–Sb substitutions. Results are given in Table 2.
Table 2. Compositional data (wt.%) from electron microprobe analysis of tetrahedrite-(Cu) (n = 17).

(σ) – estimated standard deviation.
X-ray diffraction data
Single-crystal X-ray diffraction intensity data were collected on an anhedral grain of tetrahedrite-(Cu), 60×40×30 μm in size, using a Bruker D8 Venture four-circle diffractometer equipped with an air-cooled Photon III detector, and microfocus MoKα radiation (Centro per l'Integrazione della Strumentazione Scientifica dell'Università di Pisa, Pisa, Italy). The detector-to-crystal distance was set to 38 mm. Data were collected using φ and ω scan modes, in 0.5° slices, with an exposure time of 45 s per frame. A total of 1496 frames were collected. The frames were integrated with the Bruker SAINT software package using a narrow-frame algorithm. Data were corrected for Lorentz-polarisation, absorption and background. Unit-cell parameters, refined on the basis of the XYZ centroids of 622 reflections above 20 σI with 11.17 < 2θ < 45.81°, are a = 10.3296(15) Å, V = 1102.2(8) Å3 and space group I $\bar{4}$![]() 3m. The crystal structure of tetrahedrite-(Cu) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the atomic coordinates of Johnson and Burnham (Reference Johnson and Burnham1985). The occurrence of a racemic twin was modelled. The M(2) site was found to be split into two sub-positions, M(2a) and M(2b). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used: Cu vs □ at M(2a); Cu vs □ at M(2b); Cu vs Fe at M(1); As vs Sb at X(3); and S vs □ at S(1) and S(2) sites (where □ = vacancy). Unconstrained refinement of the site occupancy at the M(2a) and M(2b) positions results in a total Cu content of 0.51(2) + 0.242(9) × 2 = 1.008 Cu atoms, indicating that no detectable Cu-excess occurs in the sample studied. Consequently, the sum of the site occupancy factors at M(2a) and M(2b) was constrained to one. The X(3) site was found fully occupied by Sb, whereas the S(1) and S(2) sites were found fully occupied by S. For these reasons, the site occupancies at these positions were fixed to one. The anisotropic structural model converged to R 1 = 0.0347 for 261 reflections with F o > 4σ(F o) and 22 refined parameters. Details of the data collection and crystal structure refinement are reported in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4, whereas Table 5 reports selected bond distances and Table 6 the weighted bond-valence balance calculated according to the bond parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
3m. The crystal structure of tetrahedrite-(Cu) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the atomic coordinates of Johnson and Burnham (Reference Johnson and Burnham1985). The occurrence of a racemic twin was modelled. The M(2) site was found to be split into two sub-positions, M(2a) and M(2b). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used: Cu vs □ at M(2a); Cu vs □ at M(2b); Cu vs Fe at M(1); As vs Sb at X(3); and S vs □ at S(1) and S(2) sites (where □ = vacancy). Unconstrained refinement of the site occupancy at the M(2a) and M(2b) positions results in a total Cu content of 0.51(2) + 0.242(9) × 2 = 1.008 Cu atoms, indicating that no detectable Cu-excess occurs in the sample studied. Consequently, the sum of the site occupancy factors at M(2a) and M(2b) was constrained to one. The X(3) site was found fully occupied by Sb, whereas the S(1) and S(2) sites were found fully occupied by S. For these reasons, the site occupancies at these positions were fixed to one. The anisotropic structural model converged to R 1 = 0.0347 for 261 reflections with F o > 4σ(F o) and 22 refined parameters. Details of the data collection and crystal structure refinement are reported in Table 3. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 4, whereas Table 5 reports selected bond distances and Table 6 the weighted bond-valence balance calculated according to the bond parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 3. Summary of data collection conditions and refinement parameters for tetrahedrite-(Cu).

1w = 1/[σ2(F o2)+(0.0262P)2]; 2Flack (Reference Flack1983).
Table 4. Sites, fractional atom coordinates, equivalent isotropic displacement parameters (Å2), and refined (obs) and calculated (calc) mean atomic numbers for tetrahedrite-(Cu).

Table 5. Selected bond distances (in Å) for tetrahedrite-(Cu).

Table 6. Weighted bond-valence sums (in valence unit) in tetrahedrite-(Cu).

Powder X-ray diffraction data were not collected, owing to the small size of the available grains and their admixture with other phases. Table 7 reports the calculated powder X-ray diffraction pattern.
Table 7. Calculated X-ray powder diffraction data for tetrahedrite-(Cu).*

* Intensity and d hkl were calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural model given in Table 4. Only reflections with I calc > 1 are listed. The five strongest reflections are given in bold.
Results and discussions
Chemical formula
As discussed in previous papers (e.g. Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021), there are different approaches to recalculating the chemical formulae of tetrahedrite-group minerals. The two better ones normalise the number of atoms on the basis of ΣMe = 16 apfu or on the basis of (As + Sb + Te + Bi) = 4 apfu. The former approach assumes that no vacancies occur at the M(2), M(1) and X(3) sites, whereas the latter is mainly based on the results discussed by Johnson et al. (Reference Johnson, Craig and Rimstidt1986) who revealed that negligible variations in the ideal number of X(3) atoms usually occurs.
The first approach gives the chemical formula Cu11.42Zn0.26Fe0.19(Sb4.06As0.08)Σ4.14S12.99, whereas the other normalisation strategy corresponds to the formula Cu11.06Zn0.25Fe0.18(Sb3.93As0.07)Σ4.00S12.57. The simplified formula of tetrahedrite-(Cu) is Cu6Cu4(Cu2+,Zn,Fe)2(Sb,As)4S13, corresponding to the end-member formula Cu6(Cu4Cu2)Sb4S13. It corresponds to (in wt.%) Cu 45.76, Sb 29.23, S 25.01, total 100.00.
Crystal structure description
The crystal structure of tetrahedrite-(Cu) agrees with the general features of the members of the tetrahedrite isotypic group. The M(2) site is split into two sub-positions, namely M(2a) and M(2b). The former has a triangular planar coordination, whereas the latter has a flat trigonal pyramidal coordination. This feature agrees with previous studies (e.g. Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Welch et al., Reference Welch, Stanley, Spratt and Mills2018). Average bond distances are 2.253 and 2.302 Å for M(2a) and M(2b), respectively. Copper was hosted at both sub-positions.
The tetrahedrally coordinated M(1) site has an average bond distance of 2.316 Å, shorter than that observed in mixed (Cu,Zn,Fe) tetrahedral sites in tetrahedrite-group minerals (e.g. Wuensch, Reference Wuensch1964; Wuensch et al., Reference Wuensch, Takéuchi and Nowacki1966) and similar to that reported by Makovicky and Skinner (Reference Makovicky and Skinner1979) for synthetic Cu12.3Sb4S13, i.e. 2.311(4) Å. On the basis of the electron microprobe data, this site should have the occupancy (Cu0.92Zn0.05Fe0.03), corresponding to 28.96 electrons per site, to be compared with a refined mean atomic number of ~29 electrons. Taking into account the low Fe content, it is possible that Fe occurs as Fe3+ (e.g. Makovicky et al., Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003), and the actual population may be described as (Cu+0.70Cu2+0.22Zn0.05Fe3+0.03). Using the bond parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991), the following ideal distances can be calculated for tetrahedral coordination: Cu+ 2.373 Å; Cu2+ 2.116 Å; Zn2+ 2.346 Å; and Fe3+ 2.266 Å. The proposed site occupancy would correspond to a calculated <M(1)–S(1)> distance of 2.312 Å, to be compared with an observed value of 2.316 Å.
The X(3) site has an average bond distance of 2.439 Å and a refined site occupancy factor indicating a full occupancy by Sb, in agreement with electron microprobe data that indicate only a very minor replacement of Sb by As, with an As/(Sb+As) atomic ratio of 0.02.
The weighted bond-valence calculations are in Table 6, obtained using the bond-valence parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991), assuming the simplified structural formula M (2)Cu6.00M (1)[Cu4(Cu1.5Zn0.30Fe0.20)]X (3)Sb4S13.
Relationship between unit-cell parameter and chemical composition
The unit-cell parameter of tetrahedrite-(Cu) from Bankov [i.e. a = 10.3296(15) Å] is identical with that of synthetic stoichiometric Cu12Sb4S13 [a = 10.3293(6) Å] described by Pfitzner et al. (Reference Pfitzner, Evain and Petříček1997) and agrees with data of synthetic Cu12.3Sb4S13 studied by Makovicky and Skinner (Reference Makovicky and Skinner1979), where a = 10.323(1) Å. No evidence of exsolution of Cu-poor and Cu-rich domains within tetrahedrite-(Cu) were observed. On the contrary, the synthetic sample studied by Di Benedetto et al. (Reference Di Benedetto, Bernardini, Cipriani, Emiliani, Gatteschi and Romanelli2005) gave a unit-cell parameter of a = 10.383(5) Å.
The relationships between chemistry and unit-cell parameter proposed by Johnson et al. (Reference Johnson, Craig and Rimstidt1987) apparently does not correctly describe the behaviour of tetrahedrite-(Cu). Indeed, the calculated a parameter is 10.37 Å, assuming the occurrence of ≈ 1.5 Cu2+ apfu. A better fit is obtained using the relationship proposed by Charlat and Lévy (Reference Charlat and Lévy1975), obtaining a calculated a value of 10.34 Å.
Comparison between tetrahedrite-(Cu) and previous findings of Cu-rich tetrahedrites
The occurrence of tetrahedrite samples having formally divalent Cu as the dominating C-cation has been reported from some other occurrences. For instance, Cesbron et al. (Reference Cesbron, Giraud, Picot and Pillard1985) reported chemical data for sample 2 from Chizeuil, France which corresponds to the empirical formula Cu6[Cu4(Cu0.74Fe0.71Zn0.42)Σ1.87](Sb2.58As1.53Bi0.01)Σ4.12S13.46. Repstock et al. (Reference Repstock, Voudouris and Kolitsch2015) documented Cu contents up to 11.12 apfu (analysis 15) in specimens from the Pefka deposit, Northeastern Greece, corresponding to the empirical formula Cu6[Cu4(Cu1.12Zn0.88Fe0.09)Σ2.09](Sb2.09As1.81Te0.14)Σ4.04S13.30. Voudouris et al. (Reference Voudouris, Repstock, Spry, Frenzel, Mavrogonatos, Keith, Tarantola, Melfos, Tombros, Zhai, Cook, Ciobanu, Schaarschmidt, Rieck, Kolitsch and Falkenberg2022) described a potential Cd–Mn bearing ‘tetrahedrite-(Cu)’ with 10.94 apfu Cu as inclusions up to 10 μm across within galena from St Philippos, Greece. The occurrence of Pb- and Cd-bearing tetrahedrite-(Cu) in association with tetrahedrite-(Cd) was mentioned by Sejkora et al. (Reference Sejkora, Biagioni, Škácha, Musetti, Kasatkin and Nestola2023) at the Radětice deposit near Příbram, Czech Republic.
Natural members of the tetrahedrite series are usually characterised by the formula Cu6(Cu4Me 2)Sb4S13, where Me is commonly Fe and Zn. However, synthetic Cu12Sb4S13 is reported in some cases to have Cu excess up to 14 apfu (e.g. Skinner et al., Reference Skinner, Luce and Makovicky1972; Tatsuka and Morimoto, Reference Tatsuka and Morimoto1973; Lind and Makovicky, Reference Lind and Makovicky1982; Makovicky and Karup-Møller, Reference Makovicky and Karup-Møller1994). Unit-cell variation from 10.323 to 10.449 Å was reported for exsolved synthetic phases with compositions ~Cu12.3Sb4S13 and ~Cu13.8Sb4S13, respectively (Makovicky and Skinner, Reference Makovicky and Skinner1979). It should also be taken into account that Lind and Makovicky (Reference Lind and Makovicky1982) highlighted an analytical problem during electron microprobe analysis of synthetic tetrahedrite-group phases; indeed, those compositions having Cu > 12 apfu gave the same analytical results as those having 12 Cu apfu. This effect was noted for both Sb- and As-members of this sulfosalt group.
Nomenclature issues in Cu-rich tetrahedrite
Type material of tetrahedrite-(Cu) from Bankov (grain used for single-crystal study) has a chemical composition close to Cu11.50Zn0.30Fe0.20Sb4.00S13 = M (2)Cu6.00M (1)[Cu4(Cu1.50Zn0.30Fe0.20)]X (3)Sb4S13. Following Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a), this chemistry can be idealised to the end-member formula Cu+10Cu2+2Sb4S13, assuming that formally divalent Cu2+ is the most abundant C constituent.
However, a majority of the chemical analyses of tetrahedrite-(Cu) and -(Fe) from Bankov (Figs. 4 and 5) are close to the ideal formula M (2)Cu6.00M (1)[Cu4(Cu1.00Fe1.00)]X (3)Sb4S13. This opens up a question of the valence of Fe. For the case with the presence of Fe3+, applying the site-total-charge approach (Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019) to this chemical composition, the end-member formula Cu6(Cu+5Fe3+)Sb4S13 = Cu11Fe3+Sb4S13 is achieved. After initial examinations in the 1970s, the first detailed 57Fe-Mössbauer studies were performed on Fe-bearing tetrahedrite in the 1990s (Charnock et al., Reference Charnock, Garner, Pattrick and Vaughan1989; Makovicky et al., Reference Makovicky, Forcher, Lottermoser and Amthauer1990 and references herein), and completed by Nasonova et al. (Reference Nasonova, Presniakov, Sobolev, Verchenko, Tsirlin, Wei, Dikarev and Shevelkov2016) and Sobolev et al. (Reference Sobolev, Presniakov, Nasonova, Verchenko and Shevelkov2017). Iron-bearing synthetic tennantite was studied by Makovicky et al. (Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003). Though first studies confirm major Fe2+ towards the Fe pole, and major Fe3+ towards the Cu pole, examination of tennantite indicates the presence of Fe2+ down to 0.5 Fe apfu, as well as mixed valence Fe. Mixed valence iron seems to represent a substantial fraction of total iron at room T, owing to charge-transfer phenomena between Cu and Fe. For instance, at a content of 0.5 Fe apfu, Makovicky et al. (Reference Makovicky, Tippelt, Forcher, Lottermoser, Karup-Møller and Amthauer2003) estimated a formal valence ranging between +2.68 and +2.69 (+2.68 for sample 2052). The oxidation state of Cu was determined by Pattrick et al. (Reference Pattrick, van der Laan, Vaughan and Henderson1993) and Gainov et al. (Reference Gainov, Dooglav, Pn'kov, Mukamedshin, Savinkov and Mozgova2008) on natural tetrahedrite and tennantite, and by Di Benedetto et al. (Reference Di Benedetto, Bernardini, Cipriani, Emiliani, Gatteschi and Romanelli2005) on synthetic Cu12Sb4S13. These three studies revealed the presence of divalent Cu in all Cu-rich samples. Nevertheless, while Di Benedetto et al. (Reference Di Benedetto, Bernardini, Cipriani, Emiliani, Gatteschi and Romanelli2005) proposed two Cu2+ apfu, located at the Cu1 [= M(1)] site, Pattrick et al. (Reference Pattrick, van der Laan, Vaughan and Henderson1993), confirmed by Gainov et al. (Reference Gainov, Dooglav, Pn'kov, Mukamedshin, Savinkov and Mozgova2008), indicates Cu2+ located at the Cu2 [= M(2)] triangular site, sometimes present for compositions excluding it according to the ionic model. Moreover, in normal conditions, pure Cu12Sb4S13 and Cu12Sb4S13 are metallic (Lu and Morelli, Reference Lu and Morelli2013), that would correspond to partial replacement of Cu2+ by Cu+ and one ligand hole (i.e. a mobile S electron).

Figure 4. Chemical composition of tetrahedrite-(Cu) and tetrahedrite-(Fe) from Bankov in a ternary Fe–Cu*–Zn graph (at. units). Cu* = contents above 10 apfu.

Figure 5. Chemical composition of tetrahedrite-(Cu) and tetrahedrite-(Fe) from Bankov in ternary Fe-Cu* graph (apfu). Cu* = contents above 10 apfu.
Thus, the solid solution from the Fe-pole to the Cu-pole would ideally correspond to the following sequence (‘ionic’ model): (1) Cu+10Fe2+2 → (2) Cu+10.5Fe2+Fe3+0.5 → (3) Cu+11Fe3+ → (4) Cu+10.5Cu2+Fe3+0.5 → (5) Cu+10Cu2+2. Compositions (1) to (3) correspond to the substitution rule 2Fe2+ → Cu+ + Fe3+, and compositions (3) to (5) to Cu+ + Fe3+ → 2Cu2+. This sequence, controlled by an increase of $f_{\rm S_2}$![]() , indicates that iron oxidation precludes the appearance of formally divalent copper. According to nomenclature rules, one should distinguish three species: (i) ‘tetrahedrite-(Fe2+)’, from formula (1) up to formula (2); (ii) ‘tetrahedrite-(Fe3+)’, from formula (2) up to formula (4); and (iii) ‘tetrahedrite-(Cu2+)’, from formula (4) up to formula (5). This is in agreement with discussions by Biagioni et al. (Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022) for the As-isotype tennantite-(Cu).
, indicates that iron oxidation precludes the appearance of formally divalent copper. According to nomenclature rules, one should distinguish three species: (i) ‘tetrahedrite-(Fe2+)’, from formula (1) up to formula (2); (ii) ‘tetrahedrite-(Fe3+)’, from formula (2) up to formula (4); and (iii) ‘tetrahedrite-(Cu2+)’, from formula (4) up to formula (5). This is in agreement with discussions by Biagioni et al. (Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022) for the As-isotype tennantite-(Cu).
On this basis, the prevailing composition of tetrahedrite from the Bankov deposit (Figs 4 and 5) falls in the field of ‘tetrahedrite-(Fe3+)’. Nevertheless, studies of natural and synthetic samples of tetrahedrite-(Cu) as well as tennantite-(Cu) through various physical methods revealed a very complex crystal chemistry, not completely understood up to now.
It thus appears that in Cu-rich tetrahedrite/tennantite one may have coexistence of Fe3+, Fe2+, Cu2+ and Cu+ (with ligand hole). The distinction between the three species envisaged above on the basis of a simple ionic model is not pertinent, and it is more convenient for nomenclature purposes to consider only two species, tetrahedrite-(Fe) and tetrahedrite-(Cu). The same solution of nomenclature was published for the analogous pair tennantite-(Cu)/tennantite-(Fe) (Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Marcoux, Mauro and Dolníček2022).
Conclusion
The description of tetrahedrite-(Cu) adds further complexity to the tetrahedrite group, confirming on one side the structural plasticity of these chalcogenides, hosting several metals typical of hydrothermal settings, and on the other their role in recording the crystallisation conditions of ore assemblages.
In addition to improving the knowledge of ore mineralogy, the description of this new phase gives further information about the crystal chemistry of tetrahedrite-group minerals, with possible technological implications, as revealed by several recent studies focusing on their high-tech properties (e.g. Suekuni et al., Reference Suekuni, Tomizawa, Ozaki and Koyano2014; Chetty et al., Reference Chetty, Bali and Mallik2015; Levinsky et al., Reference Levinsky, Candolfi, Dauscher, Tobola, Hejtmánek and Lenoir2019; Rout et al., Reference Rout, Tippireddy, Kumari, Dasgupta and Mallik2023). Among the chemical compositions showing interesting properties, synthetic Cu12Sb4S13 has potential electronic and photovoltaic properties and for this reason has been the focus of several research projects in the last decade (e.g. Tamilselvan and Bhattacharyya, Reference Tamilselvan and Bhattacharyya2018; Liu et al., Reference Liu, Chen, Mei, Hu, Yang and Chen2019, Reference Liu, Zhao, Yang, Li, Wei, Hu and Chen2020; Long et al., Reference Long, Peng, Huang, Wang, Luo, Fu, Chen and Chen2022; Mukherjee et al., Reference Mukherjee, Voneshen, Duff, Goddard, Powell and Vaqueiro2023; Lim et al., Reference Lim, Li, Zhang, Wu, Zhou, Wang, Yang, Liu, Wang, Wong, Ng, Liu and Cabot2024).
Acknowledgements
The helpful comments of an anonymous reviewer, Panagiotis Voudouris and Principal Editor Stuart Mills are greatly appreciated. JS acknowledges financial support from the Ministry of Culture of the Czech Republic (long-term project DKRVO 2024-2028/1.II.a; National Museum, 00023272). The study was also financially supported by the Ministero dell'Istruzione, dell'Università e della Ricerca through the project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32 for CB, and by the Slovak Research and Development Agency under the contract APVV-22-0041 and VEGA 2/0029/23 for JS and MS. The Centro per l'Integrazione della Strumentazione scientifica dell'Università di Pisa (C.I.S.U.P.) is acknowledged for the access to the C.I.S.U.P. X-ray Laboratory.
Competing interests
The authors declare none.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2024.24.