Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Han, Jangmi
Brearley, Adrian J.
and
Keller, Lindsay P.
2015.
Microstructural evidence for a disequilibrium condensation origin for hibonite‐spinel inclusions in the ALHA77307 CO3.0 chondrite.
Meteoritics & Planetary Science,
Vol. 50,
Issue. 12,
p.
2121.
Singh, Vijay
Borkotoky, S.
Murali, A.
Rao, J.L.
Gundu Rao, T.K.
and
Dhoble, S.J.
2015.
Electron paramagnetic resonance and photoluminescence investigation on ultraviolet-emitting gadolinium-ion-doped CaAl12O19 phosphors.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 139,
Issue. ,
p.
1.
Li, Jun
Medina, Elena A.
Stalick, Judith K.
Sleight, Arthur W.
and
Subramanian, M.A.
2016.
Colored oxides with hibonite structure: A potential route to non-cobalt blue pigments.
Progress in Solid State Chemistry,
Vol. 44,
Issue. 4,
p.
107.
Ardit, Matteo
Borcănescu, Silvana
Cruciani, Giuseppe
Dondi, Michele
Lazău, Ioan
Păcurariu, Cornelia
Zanelli, Chiara
and
McKittrick, J.
2016.
Ni‐Ti Codoped Hibonite Ceramic Pigments by Combustion Synthesis: Crystal Structure and Optical Properties.
Journal of the American Ceramic Society,
Vol. 99,
Issue. 5,
p.
1749.
Singh, Vijay
Watanabe, S.
Rao, T. K. Gundu
Singh, N.
Srivastava, Anoop K.
Singh, Pramod K.
Gao, H.
Mardina, P.
and
Dhoble, S. J.
2016.
Photoluminescence and ESR identification of γ-ray irradiation induced defects responsible for thermoluminescence in Tb3+ activated hibonite (CaAl12O19) powder material.
Journal of Electroceramics,
Vol. 37,
Issue. 1-4,
p.
58.
Pankin, I. A.
Kravtsova, A. N.
Polozhentsev, O. E.
and
Soldatov, A. V.
2016.
Modelling of substitutional defects in the structure of Ti-bearing hibonite.
Journal of Structural Chemistry,
Vol. 57,
Issue. 7,
p.
1369.
Li, Jun
Medina, Elena A.
Stalick, Judith K.
Sleight, Arthur W.
and
Subramanian, M.A.
2016.
Structural studies of CaAl12O19, SrAl12O19, La2/3+δ Al12–δO19, and CaAl10NiTiO19 with the hibonite structure; indications of an unusual type of ferroelectricity.
Zeitschrift für Naturforschung B,
Vol. 71,
Issue. 5,
p.
475.
Medina, Elena A.
Li, Jun
and
Subramanian, M.A.
2017.
Colored oxides with hibonite structure II: Structural and optical properties of CaAl12O19-type pigments with chromophores based on Fe, Mn, Cr and Cu.
Progress in Solid State Chemistry,
Vol. 45-46,
Issue. ,
p.
9.
Krzątała, Arkadiusz
Panikorovskii, Taras L.
Galuskina, Irina O.
and
Galuskin, Evgeny V.
2018.
Dynamic Disorder of Fe3+ Ions in the Crystal Structure of Natural Barioferrite.
Minerals,
Vol. 8,
Issue. 8,
p.
340.
Singh, Vijay
Sivaramaiah, G.
Singh, N.
Rao, J. L.
Dubey, Vikas
and
Singh, Pramod K.
2018.
Thermodynamic and magnetic properties of Fe doped CaAl12O19 material prepared by combustion route and post-heat treatment.
Journal of Materials Science: Materials in Electronics,
Vol. 29,
Issue. 8,
p.
6579.
Sharygin, Victor V.
2019.
A hibonite-spinel-corundum-hematite assemblage in plagioclase-clinopyroxene pyrometamorphic rocks, Hatrurim Basin, Israel: mineral chemistry, genesis and formation temperatures.
Mineralogical Magazine,
Vol. 83,
Issue. 1,
p.
123.
Han, Jangmi
Keller, Lindsay P.
Liu, Ming-Chang
Needham, Andrew W.
Hertwig, Andreas T.
Messenger, Scott
and
Simon, Justin I.
2020.
A coordinated microstructural and isotopic study of a Wark-Lovering rim on a Vigarano CAI.
Geochimica et Cosmochimica Acta,
Vol. 269,
Issue. ,
p.
639.
Holtstam, Dan
and
Hålenius, Ulf
2020.
Nomenclature of the magnetoplumbite group.
Mineralogical Magazine,
Vol. 84,
Issue. 3,
p.
376.
Krüger, Biljana
Galuskin, Evgeny V.
Galuskina, Irina O.
Krüger, Hannes
and
Vapnik, Yevgeny
2021.
Kahlenbergite KAl<sub>11</sub>O<sub>17</sub>, a new <i>β</i>-alumina mineral and Fe-rich hibonite from the Hatrurim Basin, the Negev desert, Israel.
European Journal of Mineralogy,
Vol. 33,
Issue. 4,
p.
341.
Asaduzzaman, Abu
Muralidharan, Krishna
and
Zega, Thomas J.
2021.
Density Functional Theory Driven Analysis of the Interplay among Structure, Composition, and Oxidation State of Titanium in Hibonite, Spinel, and Perovskite.
ACS Earth and Space Chemistry,
Vol. 5,
Issue. 3,
p.
544.
Ardit, Matteo
Cámara, Fernando
and
Hålenius, Ulf
2021.
Vanadium-induced coloration in grossite (CaAl4O7) and hibonite (CaAl12O19).
American Mineralogist,
Vol. 106,
Issue. 4,
p.
599.
Han, Jangmi
Ohnishi, Ichiro
Yasuhara, Akira
and
Keller, Lindsay P.
2022.
Atomic-scale structure and non-stoichiometry of meteoritic hibonite: A transmission electron microscope study.
American Mineralogist,
Vol. 107,
Issue. 5,
p.
873.
Zanetta, Pierre-Marie
Manga, Venkateswara Rao
Chang, Yao-Jen
Ramprasad, Tarunika
Weber, Juliane
Beckett, John R.
and
Zega, Thomas J.
2023.
Atomic-scale characterization of the oxidation state of Ti in meteoritic hibonite: Implications for early solar system thermodynamics.
American Mineralogist: Journal of Earth and Planetary Materials,
Vol. 108,
Issue. 5,
p.
881.
Desch, Steven J.
Dunham, Emilie T.
Herbst, Ashley K.
Unterborn, Cayman T.
Sharp, Thomas G.
Bose, Maitrayee
Mane, Prajkta
and
Williams, Curtis D.
2023.
Origin of Low-26Al/27Al Corundum/Hibonite Inclusions in Meteorites.
The Astrophysical Journal,
Vol. 953,
Issue. 2,
p.
146.
Guerch, Mhamed Ali
Jawed, Mubeen
Tariq, Ghaus
Jawed, Ahsan
Mistry, Milap
and
Asaduzzaman, Abu
2024.
Compositional-Dependent Structural Parameters and Energetics of Hibonite: Quantum-Chemical Investigation.
The Journal of Physical Chemistry A,
Vol. 128,
Issue. 28,
p.
5605.
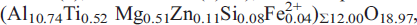 was investigated between temperatures of 100 K and 923 K by single-crystal X-ray diffraction methods. Structure refinements have been performed at 100, 296, 473 and 923 K. In hibonite from Myanmar, Ti substitutes for Al mainly at the octahedral Al4 site and, to a lesser degree, at the trigonal bipyramidal site, Al2. The Al4 octahedra build face-sharing dimers. If Ti4+ substitutes at Al4, adjacent cations repulse each other for electrostatic reasons, leading to off-centre cation displacement associated with significant bond-length distortion compared to synthetic (Ti-free) CaAl12O19. Most Mg and smaller proportions of Zn and Si are assigned to the tetrahedral Al3 site. 12-coordinated Ca in hibonite replaces oxygen in a closest-packed layer. However, Ca is actually too small for this site and engages in a ‘rattling-type’ motion with increasing temperature. For this reason, Ca does not significantly increase thermal expansion coefficients of hibonite. The expansion of natural Ti,Mg-rich hibonite between 296 and 923 K along the x and the z axes is αa = 7.64×10–6 K–1 and αc = 11.19×10–6 K–1, respectively, and is thus very similar to isotypic, synthetic CaAl12O19 and LaMgAl11O19 (LMA).
was investigated between temperatures of 100 K and 923 K by single-crystal X-ray diffraction methods. Structure refinements have been performed at 100, 296, 473 and 923 K. In hibonite from Myanmar, Ti substitutes for Al mainly at the octahedral Al4 site and, to a lesser degree, at the trigonal bipyramidal site, Al2. The Al4 octahedra build face-sharing dimers. If Ti4+ substitutes at Al4, adjacent cations repulse each other for electrostatic reasons, leading to off-centre cation displacement associated with significant bond-length distortion compared to synthetic (Ti-free) CaAl12O19. Most Mg and smaller proportions of Zn and Si are assigned to the tetrahedral Al3 site. 12-coordinated Ca in hibonite replaces oxygen in a closest-packed layer. However, Ca is actually too small for this site and engages in a ‘rattling-type’ motion with increasing temperature. For this reason, Ca does not significantly increase thermal expansion coefficients of hibonite. The expansion of natural Ti,Mg-rich hibonite between 296 and 923 K along the x and the z axes is αa = 7.64×10–6 K–1 and αc = 11.19×10–6 K–1, respectively, and is thus very similar to isotypic, synthetic CaAl12O19 and LaMgAl11O19 (LMA).

