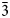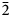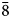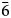Introduction
During our long-term systematic mineralogical research on the Příbram ore area in the Czech Republic (Sejkora et al., Reference Sejkora, Škácha, Plášil, Dolníček and Ulmanová2021, Reference Sejkora, Dolníček, Zachariáš, Ulmanová, Šrein and Škácha2022; Škácha et al., Reference Škácha, Sejkora and Plášil2017, Reference Škácha, Sejkora and Plášil2018, Reference Škácha, Sejkora, Plášil, Dolníček and Ulmanová2021; Dolníček et al., Reference Dolníček, Ulmanová, Sejkora, Knížek and Škácha2023), we also focused attention on the study of the unique bonanza-type silver mineralisation of hydrothermal vein B117 of the Brod deposit. As part of this research, we identified a new species, a Co-dominant member of the dolomite group. In this paper, we report its description.
The new mineral is named škáchaite in honour of Pavel Škácha (born 1981), a Czech mineralogist and curator of the mineralogical collection of the Mining Museum Příbram, Czech Republic. Pavel Škácha is the author of over sixty publications in the field of mineralogy and the history of mining, including the description of more than twelve new mineral species, especially from the Příbram ore area and from the Jáchymov ore district, Czech Republic. The new mineral and its name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2022–143, Sejkora et al., Reference Sejkora, Plášil, Dolníček and Škoda2023). Its mineral symbol, in accordance with Warr (Reference Warr2021), is Škác. The holotype material (polished section) of škáchaite is deposited in the collections of the Department of Mineralogy and Petrology, National Museum in Prague, Cirkusová 1740, 193 00 Praha 9, Czech Republic, under the catalogue number P1P 52/2022. This polished section was taken from a large sample deposited in the same collection under catalogue number P1N 49.308. Another polished section (cotype) is deposited in the collection of the Mining Museum Příbram, Czech Republic (catalogue number 1/2024).
Occurrence and mineral description
Occurrence
Škáchaite has been found in samples from the hydrothermal vein B117 between the 5th–6th level (depth 250–300 m below surface) of shaft No. 6 – Brod southeast of Příbram. In the period 1950–1991, this mine was one of the shafts open to the shallow parts of the Brod deposit, which belongs to the uranium and base-metal Příbram ore district, central Bohemia, Czech Republic (Komínek, Reference Komínek1995). The GPS coordinates for the occurrence of škáchaite are 49°40′5.094″N, 14°1′13.809″E. The uranium and base-metal Příbram ore district and the neighbouring Březové Hory ore district form the two main parts of the Příbram ore area.
The Příbram uranium and base-metal district represents the most significant accumulation of vein-type hydrothermal U ores in the Czech Republic. The hydrothermal U-mineralisation of late Variscan age is related to a 1–2 km wide and almost 25 km long zone formed by a strongly tectonised series of Upper Proterozoic sedimentary rocks along the contact with granitoids of the Devonian–Carboniferous Central Bohemian Plutonic Complex (Litochleb et al., Reference Litochleb, Černý, Litochlebová, Sejkora and Šreinová2003). A complete list of over 320 minerals identified to date from this area is given on mindat.org at http://www.mindat.org/loc-779.html.
The holotype polished section of the new mineral was taken from a museum specimen (National Museum Prague, catalogue number P1N 49.308) with dimensions 45 × 42 × 13 cm, which represents the complete profile through a 22 cm thick symmetrical vein filling, also including surrounding rock (Fig. 1). This unique sample of bonanza-type silver mineralisation contains abundant elongated native silver aggregates up to 6 cm in length in gangue formed by various types of carbonates: calcite, siderite, spherocobaltite, ankerite, kutnohorite, minrecordite and members of the dolomite–škáchaite series. Other associated minerals are safflorite, löllingite, cobaltpentlandite, Co-analogue of heazlewoodite, siegenite, carrollite, mckinstryite, jalpaite, chalcocite, tetrahedrite-(Zn), argentotetrahedrite-(Fe), not yet approved ‘argentotennantite-(Fe)’ (IMA2023-126 under voting), hematite, sphalerite, galena, chalcopyrite, pyrite and hisingerite (Litochleb et al., Reference Litochleb, Sejkora and Šrein2002). Škáchaite is of low-temperature hydrothermal origin.

Figure 1. Overall photo of symmetrical vein filling with abundant elongated native silver aggregates up to 6 cm in length in gangue formed by various types of carbonates – calcite, siderite, spherocobaltite, ankerite, kutnohorite, minrecordite and members of the dolomite–škáchaite series (catalogue number P1N 49.308, mineralogical collection of the National Museum Prague); vein B117, Brod deposit, the uranium and base-metal Příbram ore district (Czech Republic). The holotype of škáchaite was taken from a central part of the sample with bright pink carbonate aggregates.
Physical and optical properties
Škáchaite occurs as anhedral grains up to 50 μm in size and growth zones in škáchaite–dolomite zoned crystals (Fig. 2) with thickness up to 100 μm but usually only 5–20 μm. Škáchaite is pale to bright pink in colour; tiny fragments are translucent. The mineral has a white streak and vitreous lustre. It does not fluoresce under either short- or long-wave ultraviolet light. The mineral is brittle, cleavage on {10$\bar{1}$![]() 1} is perfect; the Mohs hardness cannot be measured due to the small size of škáchaite grains and may be close to 3½–4, similar to other members of the dolomite group. The density could not be measured directly because of the small amount of available material. The calculated density is 3.140 g/cm3 based on the empirical formula and unit-cell volume from single-crystal data.
1} is perfect; the Mohs hardness cannot be measured due to the small size of škáchaite grains and may be close to 3½–4, similar to other members of the dolomite group. The density could not be measured directly because of the small amount of available material. The calculated density is 3.140 g/cm3 based on the empirical formula and unit-cell volume from single-crystal data.

Figure 2. Back-scattered electron image of holotype material (P1P 52/2022) – zoned škáchaite-dolomite crystals. Škáchaite zones are white, whereas dolomite (with variable Mg/Co/Fe) ratios) is grey. The red box indicates the area used for the single-crystal X-ray diffraction study.
In thin section, škáchaite is pink with a distinct rhombohedral cleavage. It is optically uniaxial (–), with ω = 1.741(3) and ɛ = 1.535(3) (measured at 589 nm). Škáchaite is not pleochroic and has hints of undulatory extinction. The Gladstone–Dale compatibility index, 1 – (K P/K C), is –0.050 for the empirical formula and unit-cell parameters from single-crystal data, indicating good compatibility (Mandarino, Reference Mandarino1981).
Chemical composition
Chemical analyses were performed using a Cameca SX100 electron microprobe operating in wavelength-dispersive mode (15 kV, 10 nA and 3 μm beam size). The following standards and X-ray lines were used to minimise line overlaps: Co (CoKα), diopside (MgKα), hematite (FeKα), rhodonite (MnKα), wollastonite (CaKα) and ZnO (ZnKα). Peak counting times were 20 s for all elements and 10 s for each background. Contents of other measured elements (Al, Ba, Cu, Na, Ni, P, Pb, Si and Sr) were always below detection limits. Matrix correction using the PAP algorithm (Pouchou and Pichoir, Reference Pouchou and Pichoir1985) was applied to the data (measured oxides and calculated amount of CO2 resting to analytical sum of 100 wt.%). Carbonate content could not be analysed directly because of the minute amount of material available; it was confirmed by Raman spectroscopy and single-crystal structure study and calculated by stoichiometry based on the ideal content of two CO3 groups in the crystal structure of dolomite-group minerals.
The chemical composition (mean of eleven analyses) of the škáchaite grain used for the single-crystal study (holotype polished section) is given in Table 1. Individual point analyses (Supplementary Table S1) show a Co-dominant character (0.41–0.49 apfu) with Mg (0.35–0.41 apfu) and minor Fe (0.04–0.06 apfu), Mn (0.03–0.05 apfu) and Zn (up to 0.01 apfu) On the basis of Σcations = 2 atoms per formula unit (apfu), the average empirical formula is Ca1.00(Co0.45Mg0.38Ca0.08Fe0.05Mn0.03Zn0.01)Σ1.00(CO3)2. The ideal formula is CaCo(CO3)2, which requires (in wt.%) CaO 25.60, CoO 34.21, CO2 40.19, a total 100.00.
Table 1. Chemical composition (in wt.%) of škáchaite

* CO2 content was calculated on the basis of two CO3 groups present in the crystal structure of dolomite-group minerals.
S.D. – standard deviation
Škáchaite was also determined in another 26 polished sections (taken from the same museum sample) usually in thin (units to tens μm) zones in škáchaite–dolomite grains. In the chemical analyses (Supplementary Table S2), the predominant Co (0.35–0.59 apfu) is accompanied by Mg (0.17–0.44 apfu), Fe (0.03–0.31 apfu), Mn (0.01–0.12 apfu) and minor Ni and Zn up to 0.06 and 0.03 apfu, respectively.
Raman spectroscopy
The Raman spectra of škáchaite were collected in the range 50–4000 cm–1 using a DXR dispersive Raman Spectrometer (Thermo Scientific) mounted on a confocal Olympus microscope. The Raman signal was excited by an unpolarised red 633 nm He–Ne gas laser and detected by a CCD detector. The experimental parameters were: 100× objective, 10 s exposure time, 100 exposures, 50 μm pinhole spectrograph aperture and 8 mW laser power level. The eventual thermal damage of the measured points was excluded by visual inspection of the excited surface after measurement, observation of possible decay of spectral features at the start of excitation and checking for thermal downshift of Raman lines. The instrument was set up by a software-controlled calibration procedure using multiple neon emission lines (wavelength calibration), multiple polystyrene Raman bands (laser frequency calibration) and standardised white-light sources (intensity calibration). Spectral manipulations were performed using Omnic 9 software (Thermo Scientific).
The Raman spectrum of škáchaite is given in Fig. 3. The main bands observed are (in wavenumbers): 1748, 1437, 1094, 723, 294 and 173 cm–1. The experimental spectrum of škáchaite agrees very well with published Raman spectra of dolomite (e.g. Gillet et al., Reference Gillet, Biellmann, Reynard and McMillan1993; Perrin et al., Reference Perrin, Vielzeuf, Laporte, Ricolleau, Rossman and Floquet2016). The strong band at 1094 cm–1 is attributed to the symmetric stretching vibration (ν1) and the band at 1437 cm–1 to the asymmetric stretching vibration (ν3) of (CO3)2– groups. A band at 723 cm–1 is assigned to the in-plane bending vibration (ν4). The out-of-plane bending vibration (ν2) is inactive in the Raman spectrum, while its overtone (2ν2) is observed at 1748 cm–1. The bands below 300 cm–1 (294 and 173 cm–1) are attributed to lattice modes.

Figure 3. Raman spectrum of škáchaite.
X-ray diffraction data
Single-crystal X-ray studies were carried out on a thin growth zone retrieved from a larger fragment identified by wavelength-dispersive spectroscopy (WDS), which was selected to be tested on a Rigaku SuperNova single-crystal diffractometer equipped with the Atlas S2 CCD detector (microfocused MoKα radiation). The approximate dimensions of the fragment are 0.06 × 0.01 × 0.01 mm and the diffraction experiment yielded the following data: trigonal space group R $\bar{3}$![]() (no. 148), a = 4.8177(18), c = 16.093(7) Å, V = 323.5(2) Å3 and Z = 3. The c:a ratio calculated from unit cell parameters is 3.3404. The intensity data for škáchaite were processed using the Rigaku CrysAlis package, including applying an empirical multi-scan absorption correction (Rigaku, 2019). The structure was solved using the intrinsic phasing algorithm of the SHELXT program (Sheldrick, Reference Sheldrick2015). Refinement proceeded by full-matrix least-squares on F 2 using Jana2020 (Petříček et al., Reference Petříček, Palatinus, Plášil and Dušek2023). A reverse–obverse twinning (Table 2) was taken into account during the refinement in Jana (see Petříček et al., Reference Petříček, Dušek and Plášil2016). The anisotropic structural model converged to R 1 = 0.0304 for 94 reflections with F o > 3σ(F o) for 20 refined parameters, including site-occupancy refinement for Mg/Co. The final refinement returned site occupancies of Co0.503 and Mg0.497; the corresponding site scattering 19.5 e – is in line with those obtained from the WDS (20.7 e –). Details of data collection and refinement are given in Table 2. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 3 and selected bond-distances and polyhedral volumes in Table 4. Anisotropic displacement parameters are reported in the crystallographic information file (cif) deposited with the Principal Editor of Mineralogical Magazine and available as Supplementary material (see below).
(no. 148), a = 4.8177(18), c = 16.093(7) Å, V = 323.5(2) Å3 and Z = 3. The c:a ratio calculated from unit cell parameters is 3.3404. The intensity data for škáchaite were processed using the Rigaku CrysAlis package, including applying an empirical multi-scan absorption correction (Rigaku, 2019). The structure was solved using the intrinsic phasing algorithm of the SHELXT program (Sheldrick, Reference Sheldrick2015). Refinement proceeded by full-matrix least-squares on F 2 using Jana2020 (Petříček et al., Reference Petříček, Palatinus, Plášil and Dušek2023). A reverse–obverse twinning (Table 2) was taken into account during the refinement in Jana (see Petříček et al., Reference Petříček, Dušek and Plášil2016). The anisotropic structural model converged to R 1 = 0.0304 for 94 reflections with F o > 3σ(F o) for 20 refined parameters, including site-occupancy refinement for Mg/Co. The final refinement returned site occupancies of Co0.503 and Mg0.497; the corresponding site scattering 19.5 e – is in line with those obtained from the WDS (20.7 e –). Details of data collection and refinement are given in Table 2. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 3 and selected bond-distances and polyhedral volumes in Table 4. Anisotropic displacement parameters are reported in the crystallographic information file (cif) deposited with the Principal Editor of Mineralogical Magazine and available as Supplementary material (see below).
Table 2. Crystal data and refinement details for škáchaite.

Table 3. Atom coordinates, equivalent atomic displacement parameters (in Å2) and site-occupancies for škáchaite.

* BVS – sum of the bond valences (in valence units) incident upon the atomic site
Table 4. Bond-distances (in Å) and polyhedral volumes (in Å3) for škáchaite in comparison with selected members of the dolomite–škáchaite series.

Note: CD1 0.00 apfu Co, CD6 0.30 apfu Co.
*General formula AB(CO3)2, where A = Ca and B = Co and Mg.
Powder X-ray diffraction data could not be collected due to the paucity of available material. The experimental pseudo-Gandolfi scan was attempted to be collected, but the observed intensities were extremely weak. This is most probably due to the small volume of diffracting material (see the crystal dimensions). Consequently, powder X-ray diffraction data, given in Table 5, were calculated using the software PowderCell 2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) and based on the structural model given in Tables 2 and 3.
Table 5. Calculated powder X-ray diffraction data for škáchaite.*

*Intensity and d hkl (in Å) were calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) and based on the structural model given in Tables 2 and 3. Only reflections with I calc > 1 are listed. The six strongest reflections are given in bold.
Crystal structure of škáchaite
The crystal structure of škáchaite is proven (as expected) to be isotypic with dolomite. The study of Perchiazzi et al. (Reference Perchiazzi, Barton and Vignola2018) based on samples from the Tenke–Fungurume district, Democratic Republic of Congo, documented the then maximum Co substitution for Mg in dolomite corresponding to the formula Ca(Mg0.70Co0.30)(CO3)2 (crystal named CD6). The crystal fragment (Fig. 2) investigated in the current study yielded the composition Ca(Co0.503Mg0.497)(CO3)2 (and further WDS analyses have proven even more Co for some of the points measured – up to 0.59 apfu). A comparison of selected polyhedral measures and interatomic distances is given in Tables 4, 6 and 7. With the increasing Co-content in the dolomite-group minerals, both the unit cell parameters increase as well, with substantial increments in a as well as in c parameters (Figs 4 and 5). As has been already pointed out and discussed by Perchiazzi et al. (Reference Perchiazzi, Barton and Vignola2018) the AO6 octahedron is always larger than the corresponding BO6 octahedron. Increasing the Co content, the B–O bond length (2.107(5) Å for škáchaite) and BO6 polyhedral volume (12.46(1) Å3 for škáchaite) both increase, whereas the opposite behaviour is apparent for the AO6 polyhedron (Table 4). Perchiazzi et al. (Reference Perchiazzi, Barton and Vignola2018) also pointed out that with increasing Co content, the AO6 and BO6 octahedra behave distinctly: the AO6 octahedron shrinks anisotropically through the shortening of its basal edges, leading to a meagre increase of the a cell parameter. The BO6 octahedron instead expands, stretching both its ‘basal’ and ‘lateral’ edges (Table 6). The explanation for the anisotropic expansion of unit cell parameters of all ‘Co-dolomites’ (including škáchaite) known to date, is caused by the anisotropic variation of AO6 and BO6 polyhedral volumes, coupled with the presence of rigid CO3 groups lying in the (0001) plane (Perchiazzi et al., Reference Perchiazzi, Barton and Vignola2018).
Table 6. ‘Basal’- and ‘lateral’-edge dimensions (in Å) of AO6 (Ca) and BO6 (Co/Mg) polyhedra in selected members of the dolomite–škáchaite series.

*Note: CD1 0.00 apfu Co, CD6 0.30 apfu Co.
Table 7. Geometry of the CO3 groups in selected members of the dolomite–škáchaite series.

*Note: CD1 0.00 apfu Co, CD6 0.30 apfu Co.

Figure 4. The correlation of unit-cell parameter a (in Å) and Co contents (apfu); note: composition published by Perchiazzi (Reference Perchiazzi2015) is based on EDS data, only (see remarks in the text).

Figure 5. The correlation of unit-cell parameter c (in Å) and Co contents (apfu); note: composition published by Perchiazzi (Reference Perchiazzi2015) is based on EDS data, only (see remarks in text).
Discussion
Cobalt mineralogy is dominated by S and As bearing minerals, both of primary or supergene origin (Hazen et al., Reference Hazen, Hystad, Golden, Hummer, Liu, Downs, Morrison, Ralph and Grew2017). Škáchaite is only the second anhydrous Co carbonate mineral to be defined after spherocobaltite; four other known Co carbonates: comblainite, kaznakhtite, kolwezite and perchiazziite, contain hydroxyl groups or water.
Cobalt-containing dolomites have been described from several occurrences in the Central African Copperbelt (Deliens and Piret, Reference Deliens and Piret1980; Fay and Barton, Reference Fay and Barton2012; Van Langendonck et al., Reference Van Langendonck, Muchez, Dewaele, Kalubi and Cailteux2013). Their chemical composition was studied by energy-dispersive spectroscopy (EDS) analyses (Douglass Reference Douglass1992, Reference Douglass1999; Gauthier and Deliens, Reference Gauthier and Deliens1999), which found Co contents in the B site not exceeding 0.11–0.18 apfu. Sweeney et al. (Reference Sweeney, Turner and Vaughan1986) state that samples from Konkola Basin in Zambia have up to 0.08 apfu Co. Minceva-Stefanova (Reference Minceva-Stefanova1997) reported data from electron microprobe analysis performed on strongly zoned Co-rich dolomite crystals from an unknown locality with Co contents up to 0.35 apfu. A crystallographic study of cobalt-rich dolomite from Kolwezi (Democratic Republic of Congo) was performed by Perchiazzi (Reference Perchiazzi2015), who reported its chemical data with 0.17 apfu Co based on EDS data, only. A complete genetic, geochemical and mineralogical study of Co-rich carbonates from the Tenke–Fungurume District, Democratic Republic of Congo, was published by Barton et al. (Reference Barton, Yang and Barton2014), with a maximum observed Co content of 0.30 apfu; the same material was used for single-crystal structural studies (Perchiazzi et al., Reference Perchiazzi, Barton and Vignola2018).
The observed Co contents in the samples from the Brod deposit (Fig. 6) are significantly higher than those published so far (Fig. 7). We found thin (tens μm) zones with contents up to 0.59 apfu Co. The grain extracted for single-crystal X-ray diffraction shows 0.41–0.49 apfu Co contents; cobalt always prevails over Mg (Fig. 6).

Figure 6. Chemical composition of dolomite-group minerals (~1400 point analyses) from the hydrothermal vein B117 of the Brod deposit (unpublished data of authors) in the Mg–Co–M graph (M = Fe + Mn + Zn + Cu + Ni).

Figure 7. Chemical composition of Co-containing (with Co > 0.1 apfu) dolomite-group minerals in the Mg–Co–M graph (M = Fe + Mn + Zn + Cu + Ni); Příbram1 – samples from other occurrences in the Příbram uranium district (unpublished data of authors), D.R. Congo2 – samples from the Democratic Republic of Congo (unpublished data of authors), D.R. Congo3 – published data (Douglass, Reference Douglass1992, Reference Douglass1999); uknown4 – published data for unknown locality (Minceva-Stefanova, Reference Minceva-Stefanova1997); D.R. Congo5 – published data (Barton et al., Reference Barton, Yang and Barton2014); D.R. Congo6 – published data (Perchiazzi, Reference Perchiazzi2015); D.R. Congo7 – published data (Perchiazzi et al., Reference Perchiazzi, Barton and Vignola2018).
The Figs 4 and 5 show that the unit cell parameters of Co-rich dolomites and škáchaite expand with increasing Co content; the discrepancy of a sample from Kolwezi (Perchiazzi, Reference Perchiazzi2015) could be explained by the Mg/Co ratio only being measured by EDS; by the presence of a fine undetected chemical zoning; by unrecognised contamination by mineral inclusion(s) in the analysed volume; or by an error introduced by twinning on the unit cell determination (Perchiazzi et al., Reference Perchiazzi, Barton and Vignola2018).
Conclusion
Škáchaite is a new Ca-Co dominant member of the dolomite group (Table 8) together with ankerite (Ca–Fe), dolomite (Ca–Mg), kutnohorite (Ca–Mn), minrecordite (Ca–Zn) and norsethite (Ba–Mg); Strunz Class 5.AB.10. It does not correspond to any valid or invalid unnamed mineral (Smith and Nickel, Reference Smith and Nickel2007).
Table 8. Comparison of škáchaite and other Ca-dominant members of the dolomite group.

*Note: Bemp – empirical occupation of BO6 polyhedra.
Škáchaite discovery and its comparison with previously known Co-containing dolomites confirm the fundamental role played by the investigation of natural mineral assemblages to reveal the possibilities of contents of elements such as Co in the crystal structure of dolomite-group minerals. In the past, it has been reported “Cobalt does not form a stable dolomite-structured carbonate, either with other transition metal ions or with Ca2+ ” (Reeder, Reference Reeder and Reeder1983; Barton et al., Reference Barton, Yang and Barton2014) and Co-dominant dolomite has so far not been obtained in synthetic runs (e.g. Goldsmith and Northrop, Reference Goldsmith and Northrop1965; Rosenberg and Foit, Reference Rosenberg and Foit1979).
Acknowledgements
The helpful comments of an anonymous reviewer, Peter Leverett, Christian L. Lengauer, Associate Editor Owen Missen and Principal Editor Stuart Mills are greatly appreciated. The study was financially supported by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2024-2028/1.II.a; National Museum, 00023272) and the Czech Science Foundation (project 19-16218S) for JS, ZD and JU. We acknowledge CzechNanoLab Research Infrastructure supported by MEYS CR (LM2023051) for the financial support of the data collection.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2024.21.
Competing interests
The authors declare none.