Article contents
Redgillite, Cu6(OH)10(SO4)·H2O, a new mineral from Caldbeck Fells, Cumbria, England: description and crystal structure
Published online by Cambridge University Press: 05 July 2018
Abstract
Redgillite, Cu6(OH)10(SO4)·H2O, space group P21/c, a 3.155(3) Å, b 10.441(8) Å, c 19.436(16) Å, β 90.089(13)°, V = 640.2(9) Å3, Z = 2, is a new mineral from Silver Gill, Caldbeck Fells, Cumbria, England. The strongest six lines of the X-ray powder-diffraction pattern [d in Å, (I)(hkl)] are: 9.72 (90) (002), 7.11 (100) (012), 4.60 (30) (022), 4.068 (20) (023), 2.880 (30) (112,11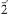 ), 2.318 (50) (131,13
), 2.318 (50) (131,13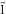 ). It occurs as translucent to transparent grass-green bladed crystals up to 0.15 mm long with squared-off or tapering terminations; usually in radiating groups. Forms observed are {001} prominent, {010} as composite stepped faces, and {100} irregular. Redgillite has white streak, vitreous lustre and Mohs hardness of ∼2. Blades are slightly flexible with irregular fracture and exhibit a perfect {001} cleavage and good {100} and {010} cleavages. The measured density (by sink-float) is 3.45(5) g/cm3; the calculated density is 3.450 g/cm3. The mineral dissolves slowly in dilute HCl. Redgillite is biaxial- negative with α = 1.693(2), β = 1.721(2), γ = 1.723(2), 2V = 30(2)° (meas.) and 30° (calc.); dispersion is r > v, medium; pleochroism: Y blue-green α X blue-green α Z yellow-green; orientation: X ≈ c , Y = b, Z ≈ a. Electron microprobe analyses yielded CuO 68.9, SO3 11.6, total 80.5. With water inferred from the structure analysis, the empirical formula is: Cu5.995(OH)9.991(SO4)1.003·H2O. Redgillite is typically found in thin fractures in partly oxidized sulphides where it is commonly associated with langite and more rarely with malachite, cuprite, connellite and brochantite. The name is for the Red Gill mine, from which the mineral is best known. The crystal structure of redgillite was determined and refined to R = 0.090 for 1529 observed reflections [I > 2σ(I)]. The redgillite structure consists of Jahn-Teller distorted CuO6 octahedra and SO4 tetrahedra. The octahedra share edges to form sheets that are zig-zag in cross section. The SO4 tetrahedra share an oxygen with the Cu layer and link the layers by hydrogen bonds to OH groups. The crystal structures of wroewolfeite, langite, posnjakite, spangolite and schulenbergite are similar to redgillite. They all contain edge sharing CuO6 layers connected to SO4 groups with the layers bridged via hydrogen bonds.
). It occurs as translucent to transparent grass-green bladed crystals up to 0.15 mm long with squared-off or tapering terminations; usually in radiating groups. Forms observed are {001} prominent, {010} as composite stepped faces, and {100} irregular. Redgillite has white streak, vitreous lustre and Mohs hardness of ∼2. Blades are slightly flexible with irregular fracture and exhibit a perfect {001} cleavage and good {100} and {010} cleavages. The measured density (by sink-float) is 3.45(5) g/cm3; the calculated density is 3.450 g/cm3. The mineral dissolves slowly in dilute HCl. Redgillite is biaxial- negative with α = 1.693(2), β = 1.721(2), γ = 1.723(2), 2V = 30(2)° (meas.) and 30° (calc.); dispersion is r > v, medium; pleochroism: Y blue-green α X blue-green α Z yellow-green; orientation: X ≈ c , Y = b, Z ≈ a. Electron microprobe analyses yielded CuO 68.9, SO3 11.6, total 80.5. With water inferred from the structure analysis, the empirical formula is: Cu5.995(OH)9.991(SO4)1.003·H2O. Redgillite is typically found in thin fractures in partly oxidized sulphides where it is commonly associated with langite and more rarely with malachite, cuprite, connellite and brochantite. The name is for the Red Gill mine, from which the mineral is best known. The crystal structure of redgillite was determined and refined to R = 0.090 for 1529 observed reflections [I > 2σ(I)]. The redgillite structure consists of Jahn-Teller distorted CuO6 octahedra and SO4 tetrahedra. The octahedra share edges to form sheets that are zig-zag in cross section. The SO4 tetrahedra share an oxygen with the Cu layer and link the layers by hydrogen bonds to OH groups. The crystal structures of wroewolfeite, langite, posnjakite, spangolite and schulenbergite are similar to redgillite. They all contain edge sharing CuO6 layers connected to SO4 groups with the layers bridged via hydrogen bonds.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2005
References
- 7
- Cited by


