Published online by Cambridge University Press: 28 February 2018
The borate mineral satimolite, which was first described in 1969 and remained poorly-studied until now, has been re-investigated (electron microprobe analysis, single-crystal and powder X-ray diffraction studies, crystal-structure determination, infrared spectroscopy) and redefined based on the novel data obtained for the holotype material from the Satimola salt dome and a recently found sample from the Chelkar salt dome, both in North Caspian Region, Western Kazakhstan. The revised idealized formula of satimolite is KNa2(Al5Mg2)[B12O18(OH)12](OH)6Cl4·4H2O (Z = 3). The mineral is trigonal, space group R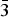 $\bar{3}$m, unit-cell parameters are: a = 15.1431(8), c = 14.4558(14) Å and V = 2870.8(4) Å3 (Satimola) and a = 15.1406(4), c = 14.3794(9) Å and V = 2854.7(2) Å3 (Chelkar). The crystal system and unit-cell parameters are quite different from those reported previously. The crystal structure of the sample from Chelkar was solved based on single-crystal data (direct methods, R = 0.0814) and the structure of the holotype from Satimola was refined on a powder sample by the Rietveld method (Rp = 0.0563, Rwp = 0.0761 and Rall = 0.0667). The structure of satimolite is unique for minerals. It contains 12-membered borate rings [B12O18(OH)12] in which BO3 triangles alternate with BO2(OH)2 tetrahedra sharing common vertices, and octahedral clusters [M7O6(OH)18] with M = Al5Mg2 in the ideal case, with sharing of corners between rings and clusters to form a three-dimensional heteropolyhedral framework. Each borate ring is connected with six octahedral clusters: three under the ring and three over the ring. Large ellipsoidal cages in the framework host Na and K cations, Cl anions and H2O molecules.
$\bar{3}$m, unit-cell parameters are: a = 15.1431(8), c = 14.4558(14) Å and V = 2870.8(4) Å3 (Satimola) and a = 15.1406(4), c = 14.3794(9) Å and V = 2854.7(2) Å3 (Chelkar). The crystal system and unit-cell parameters are quite different from those reported previously. The crystal structure of the sample from Chelkar was solved based on single-crystal data (direct methods, R = 0.0814) and the structure of the holotype from Satimola was refined on a powder sample by the Rietveld method (Rp = 0.0563, Rwp = 0.0761 and Rall = 0.0667). The structure of satimolite is unique for minerals. It contains 12-membered borate rings [B12O18(OH)12] in which BO3 triangles alternate with BO2(OH)2 tetrahedra sharing common vertices, and octahedral clusters [M7O6(OH)18] with M = Al5Mg2 in the ideal case, with sharing of corners between rings and clusters to form a three-dimensional heteropolyhedral framework. Each borate ring is connected with six octahedral clusters: three under the ring and three over the ring. Large ellipsoidal cages in the framework host Na and K cations, Cl anions and H2O molecules.
Associate Editor: Andrew Christy
Deceased