Published online by Cambridge University Press: 05 July 2018
The crystal structure and the chemical composition of a paravauxite from the Siglo Veinte Mine, Llallagua, Bustillo Province, Potosi Department, Bolivia [Fe (Fe0.916 2+Mn0.016 2+Mg0.064Ca0.002)∑0.998Al(1)Al(2)Al2.005P (P1.998Si0.002)∑2O8(OH)2·8H2O, a = 5.242(1) Å, b = 10.569(2) Å, c = 6.970(2) Å, α = 106.78(3)°, β = 110.81(2)° and γ = 72.29(2)°, space group P 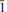 ], was investigated by single-crystal neutron diffraction and electron microprobe analysis in wavelength-dispersive mode. Neutron-intensity data were collected at 293 K and anisotropic structure refinement was performed. At the end of the refinement no peak larger than ±1.3 fm/Å3 was present in the final difference-Fourier map of the nuclear density. The final statistical index was R 1 = 0.0495 for 194 refined parameters and 1678 unique reflections with F o > 4σ(F o). Eleven independent H sites (i.e. H(1), H(2), H(3), H(4A), H(4B), H(5), H(6), H(7), H(8), H(9A) and H(9B)), all at ∼1 Å from the respective O sites, were located successfully. H(4A) and H(4B) and H(9A) and H(9B) are two mutually exclusive subsite couples only 0.4−0.6 Å apart. The complex H-bonding scheme in the paravauxite structure is now well defined and 12 independent H bonds, with an energetically favourable bonding configuration, are described. A comparison between the previous experimental findings based on Raman and infrared spectroscopy and those obtained in this present study is carried out. Paravauxite provides the rare opportunity to investigate the H-bond configuration of coexisting hydroxyl groups and H2O molecules in minerals by single-crystal neutron diffraction. H2O is present as zeolitic (i.e. lying in the cavities) and non-zeolitic H2O (i.e. bonded to Al or Fe to form Al or Fe octahedra).
], was investigated by single-crystal neutron diffraction and electron microprobe analysis in wavelength-dispersive mode. Neutron-intensity data were collected at 293 K and anisotropic structure refinement was performed. At the end of the refinement no peak larger than ±1.3 fm/Å3 was present in the final difference-Fourier map of the nuclear density. The final statistical index was R 1 = 0.0495 for 194 refined parameters and 1678 unique reflections with F o > 4σ(F o). Eleven independent H sites (i.e. H(1), H(2), H(3), H(4A), H(4B), H(5), H(6), H(7), H(8), H(9A) and H(9B)), all at ∼1 Å from the respective O sites, were located successfully. H(4A) and H(4B) and H(9A) and H(9B) are two mutually exclusive subsite couples only 0.4−0.6 Å apart. The complex H-bonding scheme in the paravauxite structure is now well defined and 12 independent H bonds, with an energetically favourable bonding configuration, are described. A comparison between the previous experimental findings based on Raman and infrared spectroscopy and those obtained in this present study is carried out. Paravauxite provides the rare opportunity to investigate the H-bond configuration of coexisting hydroxyl groups and H2O molecules in minerals by single-crystal neutron diffraction. H2O is present as zeolitic (i.e. lying in the cavities) and non-zeolitic H2O (i.e. bonded to Al or Fe to form Al or Fe octahedra).