Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Mills, S. J.
Christy, A. G.
Génin, J.-M. R.
Kameda, T.
and
Colombo, F.
2012.
Nomenclature of the hydrotalcite supergroup: natural layered double hydroxides.
Mineralogical Magazine,
Vol. 76,
Issue. 5,
p.
1289.
Mills, S. J.
Christy, A. G.
Kampf, A. R.
Housley, R. M.
Favreau, G.
Boulliard, J.-C.
and
Bourgoin, V.
2012.
Zincalstibite-9R: the first nine-layer polytype with the layered double hydroxide structure-type.
Mineralogical Magazine,
Vol. 76,
Issue. 5,
p.
1337.
Kolitsch, Uwe
Giester, Gerald
and
Pippinger, Thomas
2013.
The crystal structure of cualstibite-1M (formerly cyanophyllite), its revised chemical formula and its relation to cualstibite-1T.
Mineralogy and Petrology,
Vol. 107,
Issue. 2,
p.
171.
Richardson, Ian G.
2013.
The importance of proper crystal-chemical and geometrical reasoning demonstrated using layered single and double hydroxides.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 69,
Issue. 2,
p.
150.
Mills, S. J.
Christy, A. G.
Schnyder, C.
Favreau, G.
and
Price, J. R.
2014.
The crystal structure of camerolaite and structural variation in the cyanotrichite family of merotypes.
Mineralogical Magazine,
Vol. 78,
Issue. 7,
p.
1527.
Génin, J.-M. R.
Mills, S. J.
Christy, A. G.
Guérin, O.
Herbillon, A. J.
Kuzmann, E.
Ona-Nguema, G.
Ruby, C.
and
Upadhyay, C.
2014.
Mössbauerite, Fe63+O4(OH)8[CO3]·3H2O, the fully oxidized ‘green rust’ mineral from Mont Saint-Michel Bay, France.
Mineralogical Magazine,
Vol. 78,
Issue. 2,
p.
447.
Zhitova, E.S.
Krivovichev, S.V.
Pekov, I.V.
Yakovenchuk, V.N.
and
Pakhomovsky, Ya.A.
2016.
Correlation between the d-value and the M2+:M3+ cation ratio in Mg–Al–CO3 layered double hydroxides.
Applied Clay Science,
Vol. 130,
Issue. ,
p.
2.
Zhitova, E. S.
Popov, M. P.
Krivovichev, S. V.
Zaitsev, A. N.
and
Vlasenko, N. S.
2017.
Quintinite-1M from the Mariinsky Deposit, Ural Emerald Mines, Central Urals, Russia.
Geology of Ore Deposits,
Vol. 59,
Issue. 8,
p.
745.
Zhitova, Elena S.
Krivovichev, Sergey V.
Yakovenchuk, Viktor N.
Ivanyuk, Gregory Yu.
Pakhomovsky, Yakov A.
and
Mikhailova, Julia A.
2018.
Crystal chemistry of natural layered double hydroxides: 4. Crystal structures and evolution of structural complexity of quintinite polytypes from the Kovdor alkaline-ultrabasic massif, Kola peninsula, Russia.
Mineralogical Magazine,
Vol. 82,
Issue. 2,
p.
329.
Consani, Sirio
Balić-Žunić, Tonci
Cardinale, Anna
Sgroi, Walter
Giuli, Gabriele
and
Carbone, Cristina
2018.
A Novel Synthesis Routine for Woodwardite and Its Affinity towards Light (La, Ce, Nd) and Heavy (Gd and Y) Rare Earth Elements.
Materials,
Vol. 11,
Issue. 1,
p.
130.
Zhitova, Elena S.
Krivovichev, Sergey V.
Pekov, Igor
and
Greenwell, H. Christopher
2019.
Crystal chemistry of natural layered double hydroxides. 5. Single-crystal structure refinement of hydrotalcite, [Mg6Al2(OH)16](CO3)(H2O)4.
Mineralogical Magazine,
Vol. 83,
Issue. 02,
p.
269.
Sotiles, Anne Raquel
and
Wypych, Fernando
2023.
Trimetallic layered double hydroxide solid solutions with the compositions Zn/Co-Al, Zn/Cu-Al and Co/Cu-Al (2 M2+:1M3+), intercalated with hydrated sulfate and sodium cations.
Inorganic Chemistry Communications,
Vol. 152,
Issue. ,
p.
110698.
Sotiles, A R
and
Wypych, F
2023.
Synthesis and characterization of aluminium-rich layered double hydroxides intercalated with sulphate and alkali metal cations (Li+, Na+, K+).
Bulletin of Materials Science,
Vol. 46,
Issue. 2,
Zhitova, Elena S.
Sheveleva, Rezeda M.
Kasatkin, Anatoly V.
Zolotarev, Andrey A.
Bocharov, Vladimir N.
Kupchinenko, Anastasia N.
and
Belakovsky, Dmitry I.
2023.
Crystal Structure of Hydrotalcite Group Mineral—Desautelsite, Mg6MnIII2(OH)16(CO3)·4H2O, and Relationship between Cation Size and In-Plane Unit Cell Parameter.
Symmetry,
Vol. 15,
Issue. 5,
p.
1029.
 , with unit-cell parameters a = 5.3506(8), c = 19.5802(15) Å, V = 485.46(10) Å3 and Z = 2 determined by single crystal X-ray diffraction. The five strongest lines in the X-ray powder diffraction pattern [d in Å, (Irel), (hkl)] are as follows: 4.901, (100), (004); 4.575, (83), (011); 2.3539, (81), (11
, with unit-cell parameters a = 5.3506(8), c = 19.5802(15) Å, V = 485.46(10) Å3 and Z = 2 determined by single crystal X-ray diffraction. The five strongest lines in the X-ray powder diffraction pattern [d in Å, (Irel), (hkl)] are as follows: 4.901, (100), (004); 4.575, (83), (011); 2.3539, (81), (11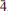 ); 1.8079, (48), (11
); 1.8079, (48), (11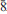 ); 3.781, (34), (103). The crystal structure was solved to R1 = 0.0896 for 356 observed reflections [Fo>4σFo] and 0.1018 for all the 469 unique reflections. Omsite is a layered double hydroxide (LDH) mineral, with a topology consistent with members of the hydrotalcite supergroup and cualstibite group.
); 3.781, (34), (103). The crystal structure was solved to R1 = 0.0896 for 356 observed reflections [Fo>4σFo] and 0.1018 for all the 469 unique reflections. Omsite is a layered double hydroxide (LDH) mineral, with a topology consistent with members of the hydrotalcite supergroup and cualstibite group.