Published online by Cambridge University Press: 24 May 2018
The new mineral novograblenovite, (NH4,K)MgCl3·6H2O, was found on basaltic lava from the 2012–2013 Tolbachik fissure eruption at the Plosky Tolbachik volcano, Kamchatka Peninsula, Russia. It occurs as prismatic, needle-like transparent crystals together with gypsum and halite. Novograblenovite was formed due to the exposure of the host rocks to eruptive gas exhalations enriched in HCl and NH3. Basalt was the source of potassium and magnesium for the mineral formation. Novograblenovite crystallises in the monoclinic space group C2/c, with unit-cell parameters a = 9.2734(3) Å, b = 9.5176(3) Å, c = 13.2439(4) Å, β = 90.187(2)°, V = 1168.91(2) Å3 and Z = 4. The five strongest reflections in the powder X-ray diffraction pattern [dobs, Å (I, %) (h k l)] are: 3.330 (100) (2 2 0), 2.976 (45) (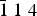 $\bar{1}\; 1\; 4$), 2.353 (29) (
$\bar{1}\; 1\; 4$), 2.353 (29) (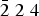 $\bar {2}\; 2\; 4$), 3.825 (26) (2 0 2), 1.997 (25) (
$\bar {2}\; 2\; 4$), 3.825 (26) (2 0 2), 1.997 (25) (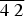 $\overline {4\; 2} $ 2). The density calculated from the empirical formula and the X-ray data is 1.504 g cm–3. The mineral is biaxial (+) with α = 1.469(2), β = 1.479(2) and γ = 1.496(2) (λ = 589 nm); 2Vmeas. = 80(10)° and 2Vcalc. = 75.7°. The crystal structure (solved and refined using single-crystal X-ray diffraction data, R1 = 0.0423) is based on the perovskite-like network of (NH4,K)Cl6-octahedra sharing chlorine vertices, and comprises [Mg(H2O)6]2+ groups in framework channels. The positions of all independent H atoms were obtained by difference-Fourier techniques and refined isotropically. All oxygen, nitrogen and chlorine atoms are involved in the system of hydrogen bonding, acting as donors or acceptors. The formula resulting from the structure refinement is [(NH4)0.7K0.3]MgCl3·6H2O. The mineral is named after Prokopiy Trifonovich Novograblenov, one of the researchers of Kamchatka Peninsula, a teacher, naturalist, geographer and geologist.
$\overline {4\; 2} $ 2). The density calculated from the empirical formula and the X-ray data is 1.504 g cm–3. The mineral is biaxial (+) with α = 1.469(2), β = 1.479(2) and γ = 1.496(2) (λ = 589 nm); 2Vmeas. = 80(10)° and 2Vcalc. = 75.7°. The crystal structure (solved and refined using single-crystal X-ray diffraction data, R1 = 0.0423) is based on the perovskite-like network of (NH4,K)Cl6-octahedra sharing chlorine vertices, and comprises [Mg(H2O)6]2+ groups in framework channels. The positions of all independent H atoms were obtained by difference-Fourier techniques and refined isotropically. All oxygen, nitrogen and chlorine atoms are involved in the system of hydrogen bonding, acting as donors or acceptors. The formula resulting from the structure refinement is [(NH4)0.7K0.3]MgCl3·6H2O. The mineral is named after Prokopiy Trifonovich Novograblenov, one of the researchers of Kamchatka Peninsula, a teacher, naturalist, geographer and geologist.
Associate Editor: G. Diego Gatta