Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Odnevall, I.
and
Leygraf, C.
1994.
The formation of Zn4SO4(OH)6·4H2O in a rural atmosphere.
Corrosion Science,
Vol. 36,
Issue. 6,
p.
1077.
Rujiwatra, Apinpus
Kepert, Cameron J.
Claridge, John B.
Rosseinsky, Matthew J.
Kumagai, Hitoshi
and
Kurmoo, Mohamedally
2001.
Layered Cobalt Hydroxysulfates with Both Rigid and Flexible Organic Pillars: Synthesis, Structure, Porosity, and Cooperative Magnetism.
Journal of the American Chemical Society,
Vol. 123,
Issue. 43,
p.
10584.
Vilminot, Serge
Richard-Plouet, Mireille
André, Gilles
Swierczynski, Dariusz
Bourée-Vigneron, Françoise
and
Kurmoo, Mohamedally
2003.
Hydrothermal Synthesis in the System Ni(OH)2−NiSO4: Nuclear and Magnetic Structures and Magnetic Properties of Ni3(OH)2(SO4)2(H2O)2.
Inorganic Chemistry,
Vol. 42,
Issue. 21,
p.
6859.
Salah, Mohsen Ben
Vilminot, Serge
André, Gilles
Bourée-Vigneron, Françoise
Richard-Plouet, Mireille
Mhiri, Tahar
and
Kurmoo, Mohamedally
2005.
Nuclear and Magnetic Structures and Magnetic Properties of Co3(OH)2(SO4)2(H2O)2. Comparison to the Mn and Ni Analogues.
Chemistry of Materials,
Vol. 17,
Issue. 10,
p.
2612.
Yoder, C. H.
Schaeffer, R. W.
McWilliams, P.
Rowand, A.
Liu, X.
and
Shambeda, J.
2011.
The synthesis of copper/zinc solid solutions of hydroxyl carbonates, sulphates, nitrates, chlorides and bromides.
Mineralogical Magazine,
Vol. 75,
Issue. 5,
p.
2573.
Rastsvetaeva, R. K.
Aksenov, S. M.
Chukanov, N. V.
and
Verin, I. A.
2012.
Crystal structure of a new mineral lahnsteinite Zn4(SO4)(OH)6 · 3H2O.
Crystallography Reports,
Vol. 57,
Issue. 5,
p.
737.
Chukanov, N. V.
Rastsvetaeva, R. K.
Aksenov, S. M.
Pekov, I. V.
Belakovskiy, D. I.
Blass, G.
and
Möhn, G.
2013.
Lahnsteinite, Zn4(SO4)(OH)6 · 3H2O, a new mineral from the Friedrichssegen Mine, Germany.
Geology of Ore Deposits,
Vol. 55,
Issue. 8,
p.
663.
Li, Peng
Li, Li
Du, Yumei
Hampton, Marc A.
Nguyen, Anh V.
Huang, Longbin
Rudolph, Victor
and
Xu, Zhi Ping
2014.
Potential foliar fertilizers with copper and zinc dual micronutrients in nanocrystal suspension.
Journal of Nanoparticle Research,
Vol. 16,
Issue. 11,
Maruyama, Swami Area
Krause, Fernanda
Filho, Sergio Rodrigues Tavares
Leitão, Alexandre Amaral
and
Wypych, Fernando
2017.
Synthesis, cation exchange and dehydration/rehydration of sodium gordaite: NaZn4(OH)6(SO4)Cl·6H2O.
Applied Clay Science,
Vol. 146,
Issue. ,
p.
100.
Stanimirova, Tsveta
Kerestedjian, Thomas
and
Kirov, Georgi
2017.
Dehydration and rehydration of Zn-hydroxy sulfate minerals with interrupted decorated hydroxide sheets.
Applied Clay Science,
Vol. 135,
Issue. ,
p.
16.
Delcheva, Zlatka
Stanimirova, Tsveta
Petrova, Nadia
and
Tacheva, Elena
2019.
Thermal decomposition of bromine gordaite: NaZn4(OH)6(SO4)Br·6H2O.
Journal of Thermal Analysis and Calorimetry,
Vol. 138,
Issue. 3,
p.
2233.
Stanimirova, Tsveta
2019.
Exchange reactions of zinc hydroxide-sulfate minerals in halide solutions.
Applied Clay Science,
Vol. 168,
Issue. ,
p.
396.
Nowinska, Katarzyna
2020.
Mineralogical and Chemical Characteristics of Slags from the Pyrometallurgical Extraction of Zinc and Lead.
Minerals,
Vol. 10,
Issue. 4,
p.
371.
Nakagaki, Shirley
Machado, Guilherme Sippel
Stival, João Felipe
Henrique dos Santos, Everton
Silva, Gabriel Machado
and
Wypych, Fernando
2021.
Natural and synthetic layered hydroxide salts (LHS): Recent advances and application perspectives emphasizing catalysis.
Progress in Solid State Chemistry,
Vol. 64,
Issue. ,
p.
100335.
ALP, Fatma Burcu
GÖNEN, Mehmet
SAVRİK, Sevdiye
and
BALKÖSE, Devrim
2021.
Zinc Borate Chemical Garden and Zinc Borate Powders from Tincal Mineral and Zinc Sulfate Heptahydrate.
Journal of Boron,
Wypych, Fernando
and
de Freitas, Rilton Alves
2022.
Clay Minerals and Synthetic Analogous as Emulsifiers of Pickering Emulsions.
Vol. 10,
Issue. ,
p.
317.
Delcheva, Zlatka
Stanimirova, Tsveta
and
Petrova, Nadia
2022.
Order of osakaite–namuwite–lahnsteinite formation during alkalization of sulfate solutions.
Review of the Bulgarian Geological Society,
Vol. 83,
Issue. 2,
p.
51.
Petrov, Petko
Stanimirova, Tsveta
and
Stariradeva, Svetla
2022.
First occurrence of the mineral osakaite Zn4(OH)6(SO4)•5H2O for Bulgaria.
Review of the Bulgarian Geological Society,
Vol. 83,
Issue. 3,
p.
35.
Wypych, Fernando
and
Satyanarayana, Kestur Gundappa
2022.
Nanoparticle-Based Polymer Composites.
p.
335.
Chen, Zongkun
Fan, Qiqi
Huang, Minghua
and
Cölfen, Helmut
2023.
The Structure, Preparation, Characterization, and Intercalation Mechanism of Layered Hydroxides Intercalated with Guest Anions.
Small,
Vol. 19,
Issue. 40,
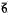 , P6/m, P622, P6mm, P
, P6/m, P622, P6mm, P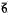 m2, P
m2, P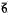 2m, and P6/mmm. Isomorphous with synthetic Zn,SO4(OH)66·4H2O and (Zn,Cu)4SO4(OH)6·4H2O. Strongest X-ray powder diffractions are: 10.59(100)0001, 5.31(15)0002, 4.15(25)11
2m, and P6/mmm. Isomorphous with synthetic Zn,SO4(OH)66·4H2O and (Zn,Cu)4SO4(OH)6·4H2O. Strongest X-ray powder diffractions are: 10.59(100)0001, 5.31(15)0002, 4.15(25)11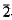 0, 2.71(42)21
0, 2.71(42)21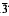 0, 2.63(41)21
0, 2.63(41)21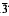 1,0004, 2.41(22)21
1,0004, 2.41(22)21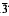 2, 1.57(23)32
2, 1.57(23)32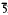 2, 1.55(20)41
2, 1.55(20)41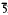 1. Namuwite is pale sea-green in colour, lustre pearly, streak very pale green, H (Mohs) 2. Cleavage {0001}, perfect. Density (g/cm3) 2.77 (meas.), 2.81 (calc. on the normalized empirical formula). It is optically uniaxial, sign not determined owing to the extremely low birefringence. Refractive index n = 1.577(5)(NaD). The mineral and name have been approved by the Commission on New Minerals and Mineral Names, IMA.
1. Namuwite is pale sea-green in colour, lustre pearly, streak very pale green, H (Mohs) 2. Cleavage {0001}, perfect. Density (g/cm3) 2.77 (meas.), 2.81 (calc. on the normalized empirical formula). It is optically uniaxial, sign not determined owing to the extremely low birefringence. Refractive index n = 1.577(5)(NaD). The mineral and name have been approved by the Commission on New Minerals and Mineral Names, IMA.